[box type=”bio”] What to Learn from this Article?[/box]
Drug-Resistant tuberculosis (MDR/XDR-TB) is increasingly being recognized as a cause of primary tuberculosis in developing countries and can be treated effectively by prolonged duration of appropriate drug-sensitivity based chemotherapy.
Case Report | Volume 7 | Issue 2 | JOCR March – April 2017 | Page 40-43| Siddharth Sanjay Shah, Aakanksha Arvind Goregaonkar, Arvind Balkrishna Goregaonkar. DOI: 10.13107/jocr.2250-0685.742
Authors: Siddharth Sanjay Shah[1], Aakanksha Arvind Goregaonkar[1], Arvind Balkrishna Goregaonkar[1]
[1] Department of Orthopedics, Lokmanya Tilak Municipal Medical College & General Hospital, Sion, Mumbai, Maharashtra. India.
Address of Correspondence
Dr. Siddharth Sanjay Shah,
G-40, 3rd Floor, Sarvodaya Nagar, 1st Panjarapole Lane, Mumbai – 400 004, Maharashtra, India.
E-mail: siddharth88@gmail.com
Abstract
Introduction: The emergence of extensively drug-resistant tuberculosis (XDR-TB) is a challenging paradigm shift faced by the TB control programs worldwide today. The treatment is further compounded with unique management difficulties faced in pediatric patients. Treatment of XDR-TB requires prolonged chemotherapy with second-line drugs which offer lesser potency and increased risk of drug-related side effects. We present a case of spinal XDR-TB in a child, managed with extended second-line antitubercular chemotherapy (ATT).
Case Report: A 6-year-old, Caucasian male child complained of persistent back pain for 3 weeks, with lumbar tenderness without neurodeficit. Radiographs showed fourth lumbar (L4) vertebral body collapse. Magnetic resonance imaging showed features suggestive of TB spondylodiscitis. The erythrocyte sedimentation rate (ESR) was raised. In view of the high prevalence rate of TB, on clinical suspicion, empirical first-line ATT (isoniazid + rifampicin + ethambutol + pyrazinamide) was given for 6 months, under the Revised National Tuberculosis Control Program (RNTCP). In the course of ATT, child developed iliopsoas abscess which was drained surgically. Repeat radiological evaluation showed almost complete L4 body destruction and 10 cm × 8 cm pre-vertebral abscess. ESR was further raised. Vertebral biopsy culture showed growth of Mycobacterium tuberculosis complex susceptible only to capreomycin (Cm). Second-line ATT (moxifloxacin + clofazimine + linezolid + isoniazid + amoxicillin-clavulanate + para-aminosalicylic [PAS] acid) with daily intramuscular Cm was initiated, under the RNTCP Programmatic Management of Drug-Resistant TB initiative. At 3 months, tenderness was absent, ESR decreased, and radiology showed consolidation of L3 and L5 vertebral bodies and their interspace. At 6 months, injectable Cm was stopped, oral ATT continued for 18 months. No major drug-related side effects were noted. At final follow-up, imaging showed complete L4 body absence, intact posterior elements, surrounding bony consolidation, resolution of abscess without neurodeficit, or deformity.
Conclusion: XDR-TB should be suspected if there is clinical and/or radiological progression of TB in spite of chemotherapy or a prior history of treatment for TB. Effective treatment of XDR-TB requires a high index of suspicion and prompt, aggressive drug sensitivity-based ATT.
Keywords: Lumbar vertebrae, extensively drug-resistant tuberculosis, antitubercular agents.
Introduction
Extensively drug-resistant tuberculosis (XDR-TB) is caused by the strain of Mycobacterium tuberculosis resistant to isoniazid and rifampicin (multidrug-resistant [MDR]-TB), with resistance to a fluoroquinolone and to at least one injectable second-line drug (amikacin, capreomycin [Cm], or kanamycin). According to the WHO 2015 report, XDR-TB has been reported in 105 countries and approximately 9.7% of MDR-TB cases are caused by XDR strains globally [1]. Treatment of XDR-TB is prolonged chemotherapy with second-line antitubercular drugs, which have a lower potency to adverse effect ratio. Children are a susceptible population for TB with unique management difficulties due to the paucibacillary nature of the infection [2] as well as due to the scarcity of child-friendly drug formulations and lack of evidence-based dosing recommendation for some drugs. We present a case of XDR-TB of the lumbar spine in a 6-year-old child, managed with drug sensitivity-based second-line antituberculous drug regimen.
Case Report
A previously asymptomatic, 6-year-old, Caucasian male child complained of insidious onset, dull aching, persistent back pain for 3 weeks, with the parents having noted weight loss, irritability, and reduced appetite in the child for a few weeks. Clinical assessment demonstrated lumbar tenderness without deformity or neurological deficit. There was no evidence of generalized lymphadenopathy, skin, abdominal, or other systemic involvement. Radiographs showed complete destruction of fourth lumbar (L4) vertebral body, sparing of the posterior elements, and focal kyphosis of L3 over L5 vertebra (Fig. 1).
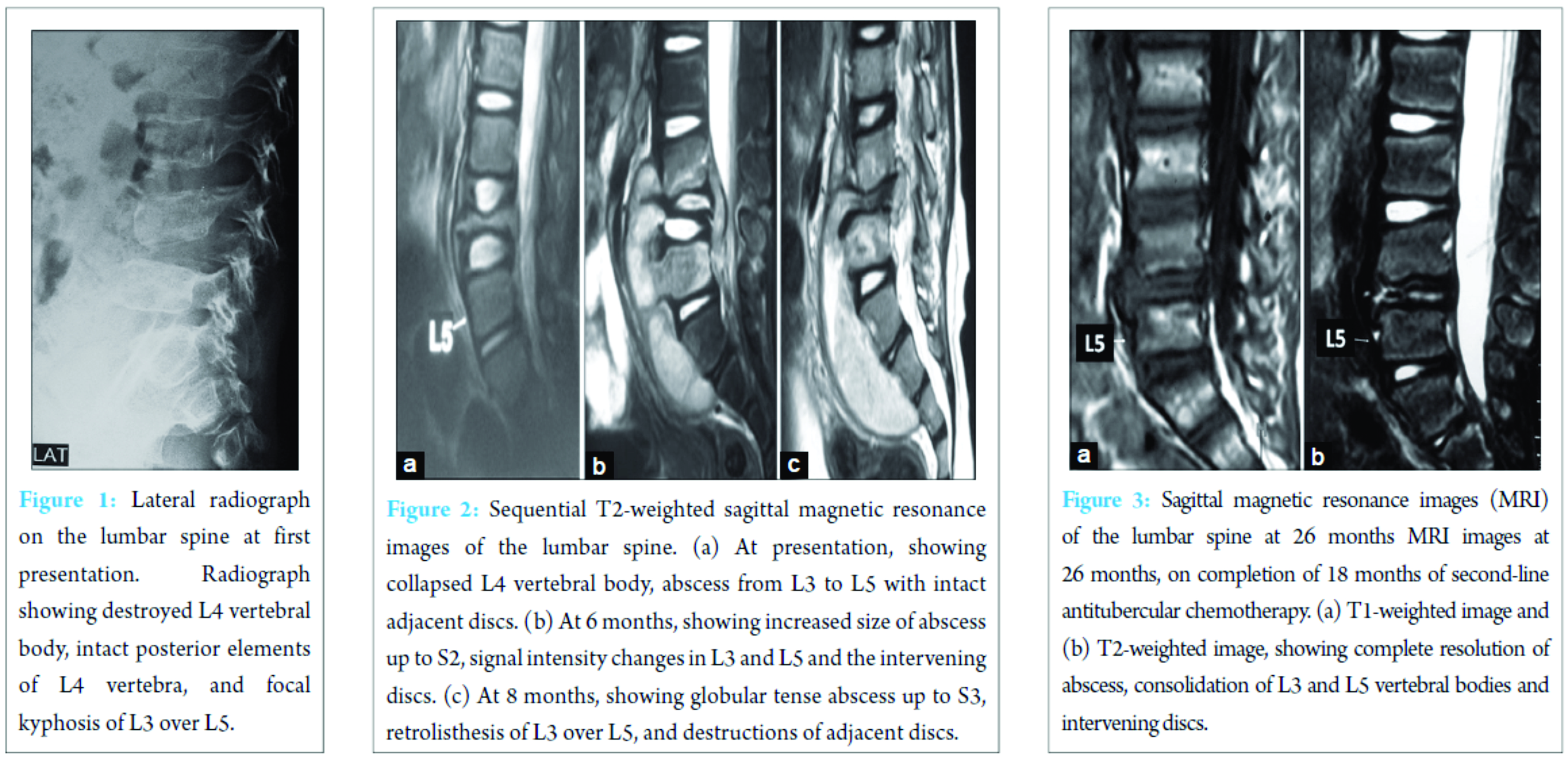
Screening radiographs of the skull, long bones of the limbs, and the rest of the spine were normal. Ultrasound of the abdomen showed 10 cm × 3 cm echo-poor iliopsoas abscess with moving internal echoes, without any organomegaly or other pathologic finding. Magnetic resonance imaging (MRI) showed features suggestive of TB spondylodiscitis with L4 vertebral body destruction, with pre-vertebral and anterior epidural abscess extending from L3 to L5 (Fig. 2). Erythrocyte sedimentation rate (ESR) was raised (40 mm/h), total white blood cell count was 12,800 (normal 4000-11,000/µL) with other blood indices being normal. The clinical and imaging findings ruled out other differential diagnoses such as Langerhans cell histiocytosis, primary or metastatic tumors such as lymphoma or Ewing’s sarcoma as well as any other cause of primary or secondary immunodeficiency. In view of high clinical suspicion, empirical first-line antituberculosis chemotherapy (ATT) was started with isoniazid, rifampicin, pyrazinamide, and ethambutol (Category 1 ATT under Revised National Tuberculosis Program [RNTCP]), given for 6 months with hip spica immobilization. In spite of clinical improvement, repeat radiological evaluation at 6 months showed further collapse of L4 body, significantly increased size of the abscess, now extending from L3 to S2, with signal intensity changes in L3 and L5 vertebral bodies (Fig. 2). Thus, ultrasound-guided percutaneous biopsy of the L4 body and aspiration of the abscess were performed under local anesthesia. Histopathology showed TB granulomatous inflammation and Ziehl–Neelsen stain was positive for acid-fast bacilli. Suspecting a resistant strain of Mycobacterium, streptomycin was added to the regimen while awaiting the culture and drug-sensitivity testing (DST) results. Culture on BACTEC MGIT 960 system using modified Middlebrook 7H9 broth, showed growth of M. tuberculosis complex strain resistant to isoniazid, rifampicin, ethambutol, pyrazinamide, streptomycin, kanamycin, ethionamide, PAS acid, and ofloxacin. The strain showed sensitivity only to Cm (critical concentration-2.5 µg/ml). Repeat radiological evaluation at 8 months, showed destruction extending to L3-4 and L4-5 discs with erosion of L3 and L5 end plates, retrolisthesis of L3 over L5, and abscess extending into the pelvic and gluteal regions (Fig. 2). ESR was raised further (60 mm/h). Second-line ATT comprising of moxifloxacin (Mfx), clofazimine (Cfz), linezolid (Lzd), high-dose isonizid (INH), amoxicillin/clavulanate (Amx/Clv), and PAS, with daily intramuscular Cm was started. At 3 months after initiation, ESR levels decreased (38 mm/hr) and radiology showed consolidation of L3 and L5 bodies and their interspace, with a kyphosis of 10° (Cobb’s method). At 6 months, injectable Cm was stopped while other oral drugs were continued. Physiotherapy and rehabilitation were started with lumbosacral bracing. Second-line ATT was given for a total duration of 18 months (26 months since first presentation to the clinic). The final radiological evaluation showed complete L4 body absence with intact posterior elements with adjacent vertebral endplate consolidation, no clinical or radiological kyphosis, no signs of spinal instability, and complete resolution of the abscess (Fig. 3 and 4). On follow-up at 6 months after completion of ATT (32 months since first presentation), the child walked comfortably, full weight bearing without any orthosis without evidence of back pain or deformity (Fig. 5).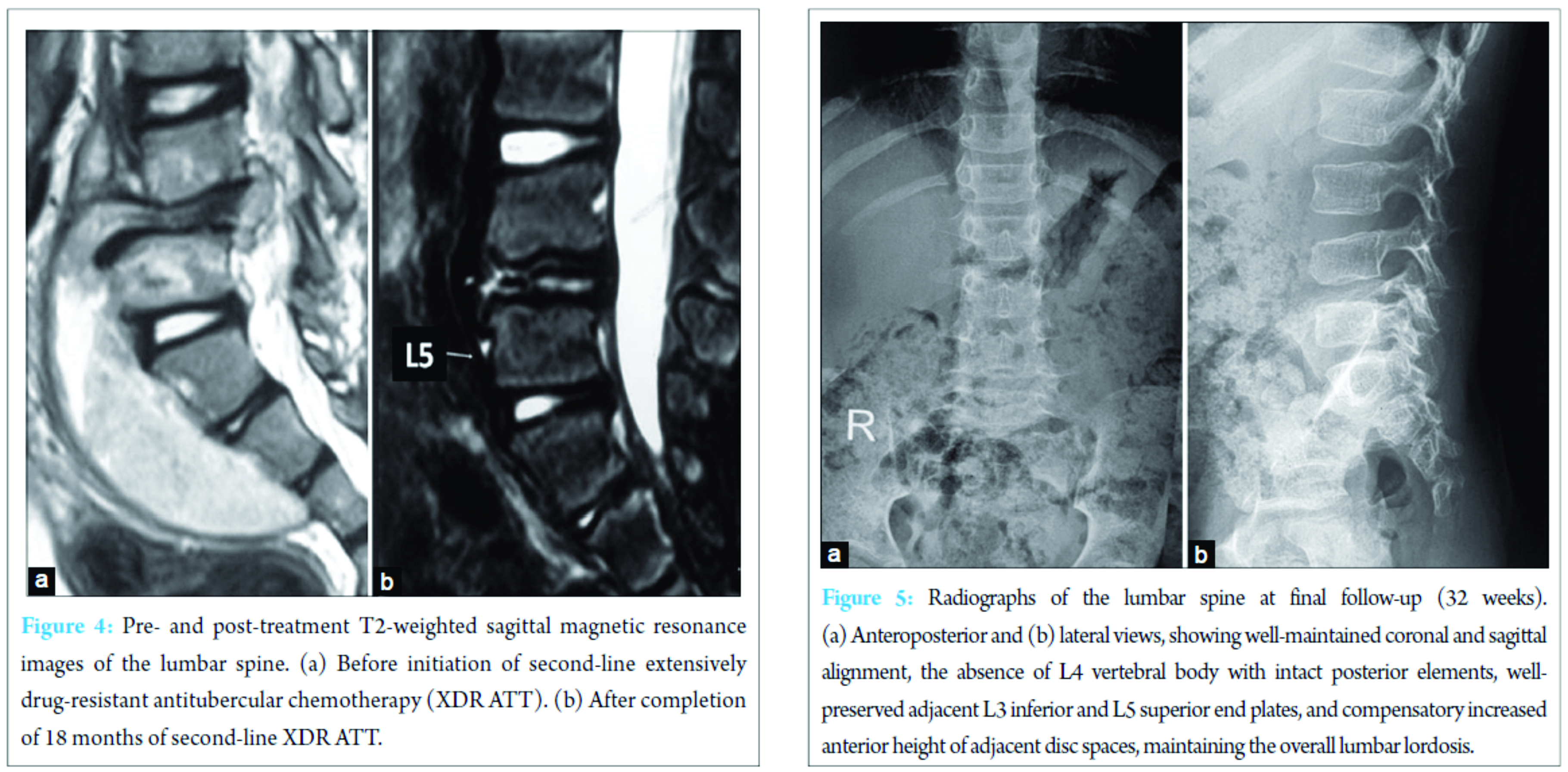
Discussion
India is an endemic region for TB infection [3]. High index of suspicion and a low threshold for initiation of empirical antituberculous treatment is usually noted among the treating physicians. However, the case highlights the fact that a DST is imperative for assessing the sensitivity pattern of the Mycobacterium strain, to detect the primary MDR or XDR-TB strains. Failure to do so leads to ineffective treatment and further emergence of drug resistance. The treatment of XDR-TB should be individualized based on prior history of TB treatment and patterns of resistance to the first- and second-line drugs. The WHO recommends special guidelines [4]: The use of pyrazinamide and/or another group 1 drug (present case – INH); the use of next-generation fluoroquinolones (Mfx or Gatifloxacin) even if DST results show resistance to levofloxacin, ofloxacin or both (present case – Mfx); the use of an injectable agent (aminoglycoside or Cm) to which the bacterial sample is susceptible (present case – Cm); the use of two or more group 5 drugs (present case – Lzd, Cfz, and Amx/Clv); and the use of all group 4 drugs that have not been extensively prescribed or that are considered effective (present case – PAS). RNTCP is the state-run TB control initiative by the Government of India. The development of Programmatic Management of Drug-Resistant Tuberculosis (PMDT) is the RNTCP’s fight against drug-resistant TB, adhering to the WHO guidelines [5]. The regimen for XDR-TB under PMDT includes 6-12 months intensive phase and 18 months continuation phase [5]. Drugs given in intensive phase includes Cm, PAS, Mxf, high-dose INH, Cfz, Lzd, and Amx/Clv, and continuation phase includes PAS, Mfx, high-dose INH, Cfz, Lzd, and Amx/Clv. The drugs to which the patient demonstrates resistance should be withdrawn and replaced with at least two new drugs to which the patient has not previously been exposed. In children, radiological imaging is used as complementary evidence of adequate treatment response. Compared with culture, which needs weeks or months, imaging is rapid and correlates well with disease progression and efficacy of TB treatments [2]. According to Zhang, follow-up MRI can clearly show the change of the vertebral body and intervertebral space, paraspinal abscess, and the kyphosis angle, which can provide reference for clinical treatment and estimating prognosis in the course of treating spinal TB of children [6]. Treatment success is dependent on several factors such as host immunity, extent of drug resistance, and disease severity. However, favorable outcomes, which are defined as cure or treatment completion, are very low (16-44%) in patients with XDR-TB [7]. Data for treatment outcomes of XDR-TB in young children are scarce, although case reports and expert opinions suggest they are likely to do better than adults [7]. Good success with medical management alone has been reported in children [8]. The successful outcome in the present case can be attributed to strict compliance, directly observed therapy, guided multidisciplinary team management, and the young age of the child.
Conclusion
This case report highlights the fact that MDR/XDR-TB should be suspected if there is clinical and/or radiological progression of TB in spite of chemotherapy and history of previous treatment for TB. Appropriate sensitivity-based chemotherapy, given ensuring good compliance to the regimen for an extended period, is the key to ensure effective treatment of XDR-TB patients.
References
1. WHO. Global Tuberculosis Report; 2015. Geneva: World Health Organization; 2015. Available from: http://www.who.int/tb/publications/global_report/en. [Last accessed on 2016 Jan 24].1. WHO. Global Tuberculosis Report; 2015. Geneva: World Health Organization; 2015. Available from: http://www.who.int/tb/publications/global_report/en. [Last accessed on 2016 Jan 24].
2. Salazar-Austin N, Ordonez AA, Hsu AJ, Benson JE, Mahesh M, Menachery E, et al. Extensively drug-resistant tuberculosis in a young child after travel to India. Lancet Infect Dis 2015;15(12):1485-1491.
3. World Health Organization. Annex 2. Country Profiles for 22 High-Burden Countries. Available from: http://www.who.int/tb/publications/global_report/gtbr15_annex02.pdf. [Last accessed on 2016 Jan 23].
4. Manjelievskaia J, Erck D, Piracha S, Schrager L. Drug-resistant TB: deadly, costly and in need of a vaccine. Trans R Soc Trop Med Hyg 2016;110(3):186-191.
5. Mishra G, Ghorpade SV, Mulani J. XDR-TB: an outcome of programmatic management of TB in India. Indian J Med Ethics 2014;11(1):47-52.
6. Zhang CB, He L, Wang YJ, He JW, Ji TT, Yan ZH. MRI-based follow-up study of spinal tuberculosis in children. Zhongguo Gu Shang 2014;27(10):878-881.
7. The Emerging Threat of Drug-Resistant Tuberculosis in Southern Africa: Global and Local Challenges and Solutions: Summary of a Joint Workshop. Available from: http://www.ncbi.nlm.nih.gov/books/NBK55582. [Last accessed on 2016 Feb 04].
8. Garcia-Prats AJ, Rose PC, Hesseling AC, Schaaf HS. Linezolid for the treatment of drug-resistant tuberculosis in children: a review and recommendations. Tuberculosis (Edinb) 2014;94(2):93-104.
 |
 |
 |
| Dr. Siddharth Shah | Dr. Aakanshka A. Goregaonkar | Dr. Arvind B. Goregaonkar |
| How to Cite This Article: Shah SS, Goregaonkar AA, Goregaonkar AB. Extensively Drug-Resistant Tuberculosis of the Lumbar Spine in a Six-year-old Child: A Case Report. Journal of Orthopaedic Case Reports 2017 Mar-Apr;7(2):40-43. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




