[box type=”bio”] Learning Points for this Article: [/box]
Pelvic hydatidosis is a rare but important differential diagnosis of tubercular hip arthritis, especially in areas with high prevalence of hydatid disease.
Case Report | Volume 7 | Issue 4 | JOCR July – August 2017 | Page 25-28| Nishit Bhatnagar, Hari Kishan, Sumit Sura, Purushotham Lingaiah, Kameshwaran Jaikumar. DOI: 10.13107/jocr.2250-0685.834
Authors: Nishit Bhatnagar[1], Hari Kishan[1], Sumit Sura[l], Purushotham Lingaiah[1], Kameshwaran Jaikumar[1]
[1] Department of Orthopaedics, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India.
Address for Correspondence
Dr. Nishit Bhatnagar,
7 Godavari Apartments, Alaknanda, New Delhi – 110019. India.
E-mail: nishitbhatnagar@yahoo.co.in
Abstract
Introduction: Cystic echinococcosis of the bone is rare and difficult to treat due to frequent recurrences, especially in certain locations such as ilium and hip, where radical surgery is difficult to achieve.
Case Report: We present a case of a 35-year-old female of the Indian subcontinent who had complaints of pain in left hip and limp since 1 year. The first clinical and radiological diagnosis was tuberculosis of the hip. However, higher imaging modalities revealed the diagnosis of hydatid disease of hip. The patient underwent surgical debridement and anti-helminthic chemotherapy. At 3-year follow-up, the patient was disease-free.
Conclusion: Hydatid disease of the hip and pelvis, although rare must be kept in the differential diagnosis of pathologies of hip-like tuberculosis. Debridement and excision of hip joint give good functional outcome, while also minimizing morbidities that are usually associated the use of custom-made prosthesis or complex arthroplasty.
Keywords: Hydatid disease, pelvis, Echinococcus.
Introduction
Hydatid cysts commonly affect the liver and the lung [1]. Cystic echinococcosis of the bone is rare with an incidence of 0.5-4% among patients with hydatid disease [2]. Bone hydatidosis is difficult to treat and carries high morbidity due to frequent recurrences, especially in certain locations such as ilium and hip, where radical surgery is difficult. We report a case of hydatid disease of hip and pelvis treated by debridement and Girdlestone arthroplasty (excision of the hip joint).
Case Report
A 35-year-old female of the Indian subcontinent, from a rural background, was referred to our center complaining of pain in left hip and limp since 1 year. The pain was insidious in onset and was associated with difficulty in squatting and sitting cross-legged. The patient had anorexia and undocumented weight loss. There was no other significant past medical history. Family history revealed that her husband had been previously treated for pulmonary tuberculosis 10 years back.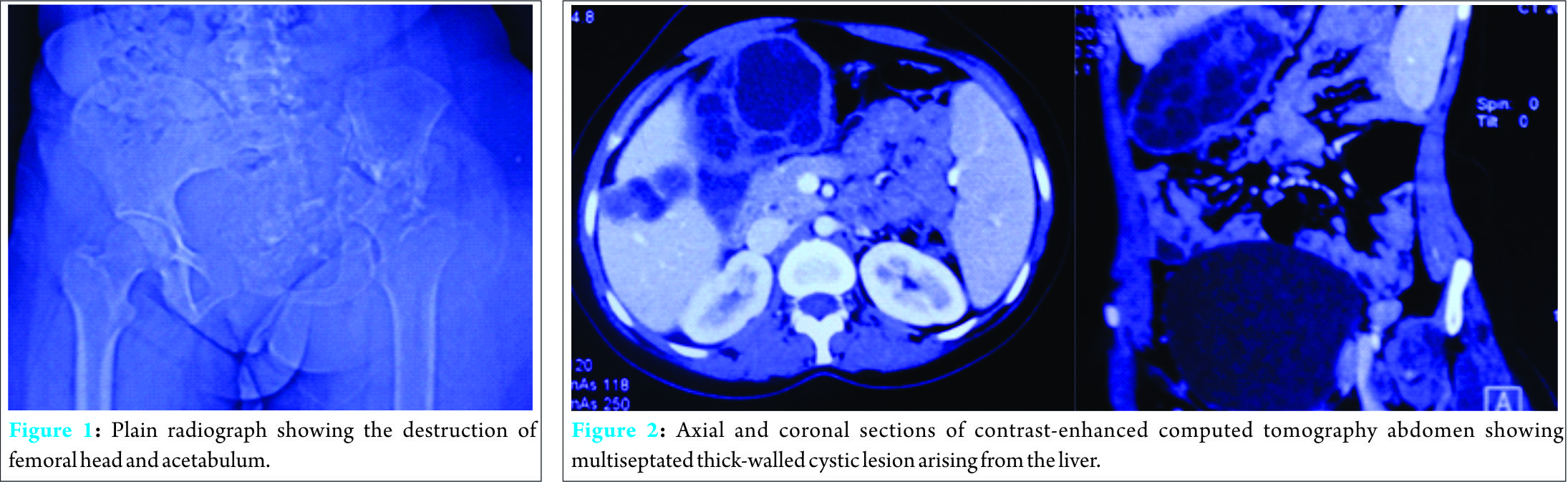
The hip was tender on palpation and associated with fullness in the iliac fossa. She had flexion deformity of 30°, with only jog of movement at the hip joint, and true shortening of 3 cm. Plain radiographs (Fig. 1) showed obliteration of joint space with the destruction of femoral head and acetabulum. Osteolytic lesions were seen in the supra-acetabular region and superior pubic ramus. Laboratory investigations revealed an elevated erythrocyte sedimentation rate (ESR) of 52 mm/h and eosinophilia, with absolute eosinophil count (AEC) of 930/mL (30-350/mL).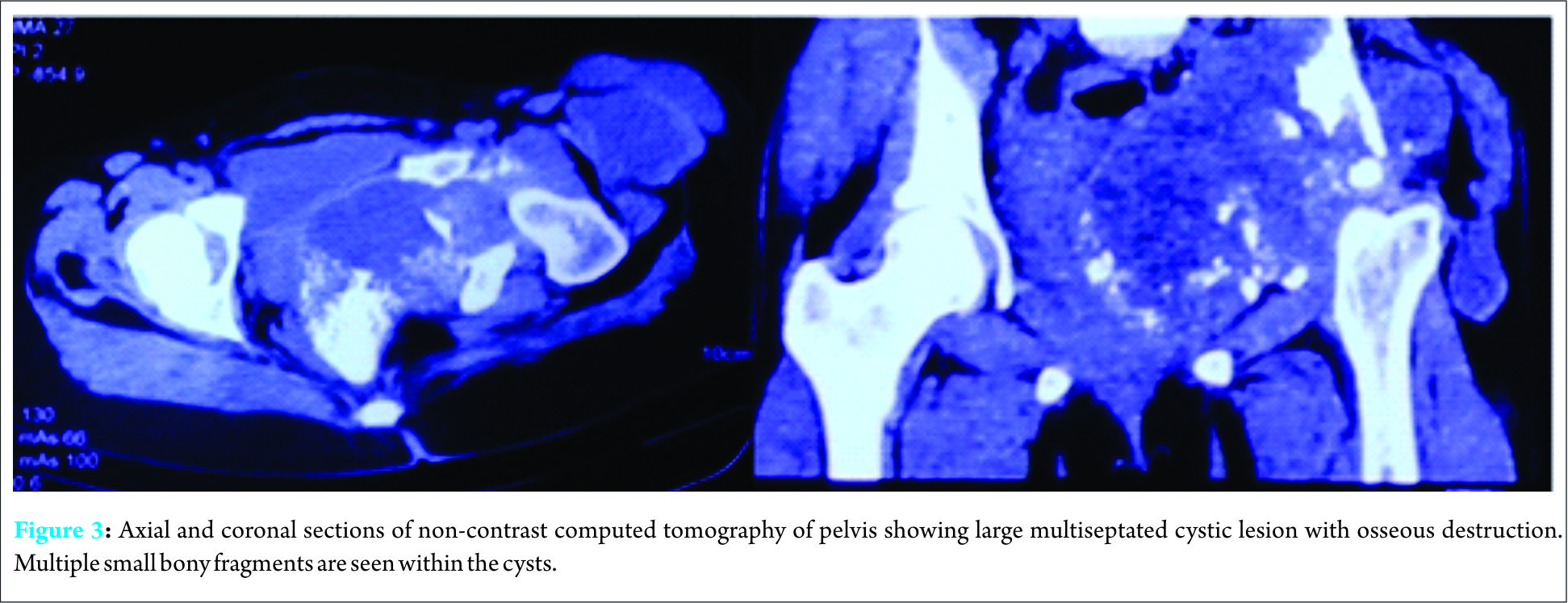
Based on this, a provisional diagnosis of tuberculosis of the hip was made. Baseline liver function tests (LFT) before starting anti-tubercular therapy (ATT) revealed mild hyperbilirubinemia (T. Bil -2.1 mg/dL) and elevated serum transaminases (alanine aminotransferase [ALT] -136 U/L, aspartate aminotransferase [AST] -145 U/L). Within 1 week of starting ATT, the patient developed abdominal pain with vomiting and icterus. LFT revealed a further rise in bilirubin levels (T. Bil -3.2 mg/dL) and serum transaminases (ALT -286 U/L, AST -312 U/L). ESR also increased to 64 mm/h and eosinophilia persisted (AEC -740/mL). ATT was withheld; however, derangement of LFT persisted.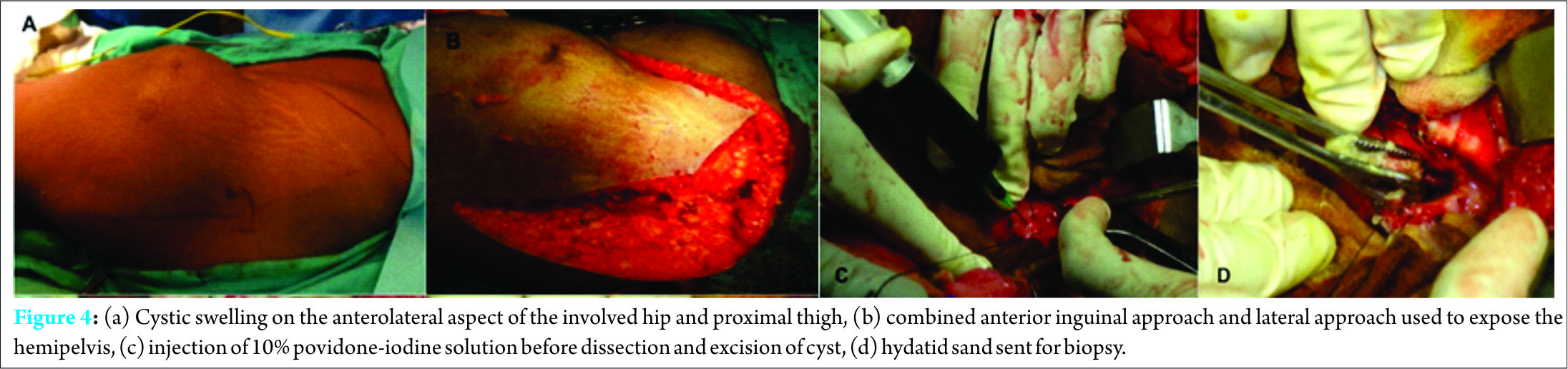
This prompted an ultrasound of the hepatobiliary system which revealed a well-circumscribed multilobulated lesion in segment V of the right lobe of the liver with peripheral daughter cysts. There was an associated large exophytic component extending medially beyond the hepatic capsule, suggestive of hydatid cyst. Henceforth, abdominal computed tomography (CT) scan with contrast (Fig. 2) was done revealing 10.2 cm × 5.6 cm exophytic multiseptated thick-walled cystic lesion arising from the right lobe of the liver. There was an associated smaller (3.3 cm × 2.3 cm) multiseptated cystic lesion with evidence of rim calcification. CT scan of the pelvis (Fig. 3) revealed a large multiseptated cystic lesion with the osseous destruction of the left iliac bone, acetabulum, and proximal femur. It showed complete destruction of the acetabulum with evidence of protrusio acetabuli. The cystic component of the lesion involved muscles around the hip joint and left hemipelvis. This extended into the ischiorectal fossa, gluteal region, and iliopsoas, with a cold abscess in pelvis tracking along the anterolateral aspect of the thigh. Multiple small bony fragments were seen in the cystic lesion.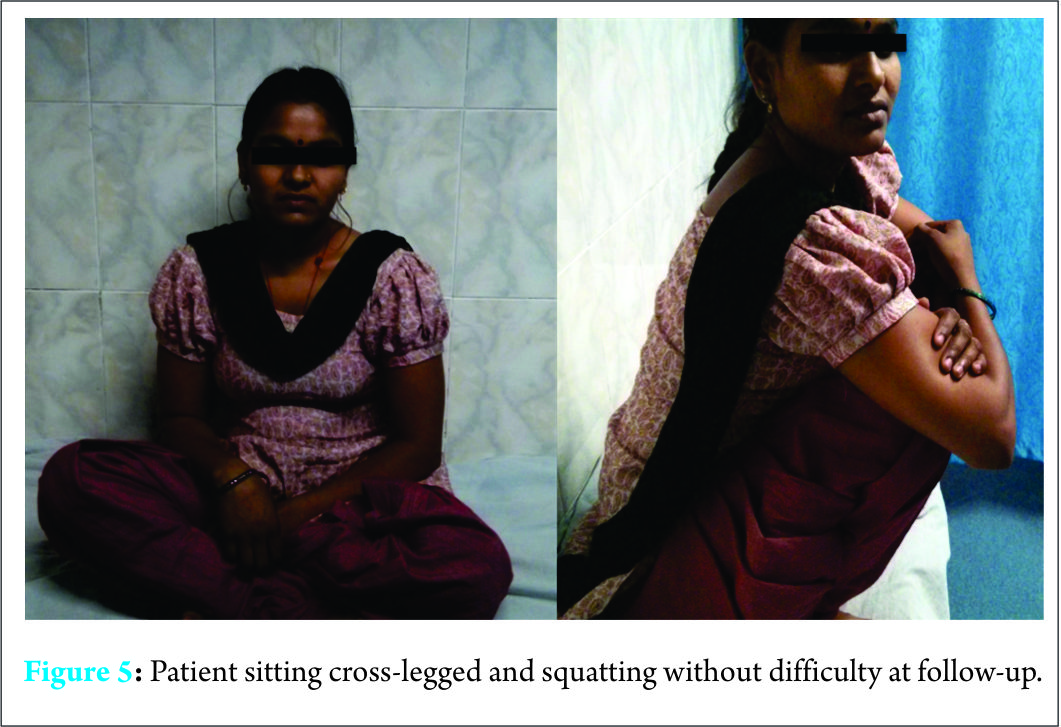
Surgical debridement was done 1 week after commencing oral treatment with albendazole 10 ?mg/kg/day. A lateral approach was used to expose the hip joint through an incision starting from the highest point of iliac crest extending distally along the posterior border of the greater trochanter (Fig. 4). On separating the fibers of gluteus medius, hydatid cyst, and debris from destroyed hip joint were encountered. The cyst was injected with 50 mL 10% povidone-iodine solution. Carefully, the extraosseous part of the cyst was dissected and excised. Using a bone saw, osteotomy of the femoral neck was made at the upper border of the lesser trochanter. The diseased and deformed femoral head was removed revealing an enlarged acetabulum filled with osseous debris, cartilage flakes and hydatid cyst. The cyst was seen to be communicating to the endopelvic. Again, 50 mL of 10% povidone-iodine solution was injected into the cyst. For endopelvic exploration, the anterior approach was made through an incision from pubic symphysis to anterior superior iliac spine and along the iliac crest to join the first incision at the highest point of the crest. The femoral neurovascular bundle was identified and preserved. Anterior abdominal wall muscles were reflected off the superior pubic ramus, inguinal ligament, and anterior portion of the anterior iliac crest. Dissection was continued in the extraperitoneal plane by retracting the peritoneum away from the affected side. Lesser pelvic wall and quadrilateral plate were identified and were observed to be involved in the parasitic infestation. Cysts were found bulging into the endopelvic from the medial acetabular wall. This cyst was communicating across the acetabulum. Careful excision of the cyst was performed. Surgical wounds were thoroughly washed with 0.9% saline and 10% povidone-iodine mixture (2:1) and closed in layers over suction drains. Fistulae were debrided and excised. No anaphylactic reaction has been observed during the surgery. Femoral head and synovial biopsy confirmed hydatid disease. Since the disease was infective in nature, we did not opt for reconstruction of the hip joint with endoprosthesis for fear of surgical site infection. Albendazole was continued for 3 months after the surgery. Skeletal traction through upper tibial Steinman pin was applied postoperatively. Gentle hip adduction/abduction skateboard exercises were initiated 2 weeks postoperatively. Hip flexion/extension exercises were added at 3 weeks. Surgical wound dehiscence occurred at 2 weeks after the surgery, but with regular dressing and oral antibiotic coverage, the wound healed within 3 weeks. At 3 months postoperatively, the patient was allowed crutch-assisted full weight-bearing ambulation. At 6-month follow-up, the patient was able to walk with the help of a stick, squat, and sit cross-legged (Fig. 5). At 3-year follow-up, the patient was walking pain-free and unaided. There was no clinical evidence of disease recurrence.
Discussion
The genus Echinococcus, commonly known as tapeworm, includes three species; Echinococcus multilocularis, Echinococcus vogeli, and Echinococcus granulosus. The larval stage of E. granulosus is the most common cause of hydatid disease in man [3].
The definitive hosts are dogs, foxes, and other carnivores. The tapeworms live in the small bowel and infected ova are shed in feces. When ingested by intermediate hosts such as man, sheep, or cattle, the larvae enter the portal circulation. Sometimes, larvae reach the lungs and other areas of the body. The life cycle is completed when the definitive hosts consume infested viscera of the intermediate host [3]. Cystic echinococcosis is a persistent zoonosis in rural livestock raising areas. Countries in the temperate zone (China, Central Asia, Mediterranean region, Australia, and parts of South America) have the greatest prevalence of cystic echinococcosis [4]. Andhra Pradesh and Tamil Nadu have the highest prevalence of hydatid disease in India [5]. Age of presentation is usually around 40 years [6, 7]. Tekin reported a female predominance [8]. However, Palanivelu reported a male predilection of the disease (5:1) [7]. The liver and lungs are the most common sites (~90%) of cysts in humans. The kidneys, spleen, and muscles are occasional sites (2-3% each). The heart, brain, and bones are extremely rare sites (<1% each) for cystic echinococcosis [9]. Vertebra accounts for the majority (75%) of osseous hydatidosis. Pelvis accounts for 16-28% of osseous hydatidosis [10]. Intraosseous invasion occurs through three methods: (1) An expansive cyst which compresses and displaces the surrounding tissues, leading to pressure-induced atrophy of the bone, (2) ischemia through obstruction or compression of the nutrient vessels, (3) osteoclast proliferation around the compressed bone tissue. Extraosseous invasion results from osseous disruption or pathologic fracture. This exogenous proliferation constitutes the hydatid abscess, which is a cold, migratory abscess, similar to a tubercular abscess [10]. Osseous hydatidosis may lie dormant for decades. It is usually detected only after a complication such as a pathologic fracture, neural deficit, and infection or fistulation of the abscess [10]. Physical findings are usually unremarkable except in a few cases. There can be a deformation of limbs, a cold or fistulated abscess, or a vertebral kyphosis. Since the disease has nonspecific features, one must maintain a high index of suspicion, especially in sheepherders, veterinarians, or butchers. The most common radiographic feature of osseous lesions is multiple expansile lucent lesions without clear boundaries, together forming a huge area of osteolysis with the classic waffle appearance [11]. This is accompanied by cortical thinning and a lack of periosteal reaction unless there is a superadded infection. Abdominal pelvic ultrasonography and radiography of the chest is required to look for a visceral hydatidosis. CT scan is the best method for diagnosis and follow-up of osseous hydatidosis. Magnetic resonance imaging is often used for defining the local extent of the lesions in the soft tissues [12]. The hydatid vesicles are seen as a hyposignal in T1-weighted images and a hypersignal in T2-weighted images [13]. The differential diagnosis includes tuberculosis, chronic osteomyelitis, and tumors such as aneurysmal cysts, osteoclastoma, chondrosarcoma, osteosarcoma, and metastases [14]. Pelvic hydatid disease is dangerous due to the clinical latency of the disease, which allows parasitic invasion of the sacroiliac and hip joint, thus making eradication of parasite is almost impossible. However, wide excision or marginal excision is preferred to decrease the parasitic load. Belzunegui et al. have shown good functional outcome after Girdlestone arthroplasty [15]. Aggarwal reported satisfactory outcome with the combination of chemotherapy and surgery [16]. Siwach et al. reported a case of extensive hydatid disease of pelvis and femur for which a hindquarter amputation was performed [17]. Prothesis is fraught with danger because of the risk of bacterial infection [18]. Wirbel reported a case where replacement was done with unsatisfactory results (Table 1) [19].
Conclusion
Hydatid disease of the hip and pelvis, although rare must be kept in the differential diagnosis of pathologies of hip-like tuberculosis. Raised eosinophil count should prompt further investigation into the cause of eosinophilia, especially if associated with deranged LFTs. Debridement and excisional arthroplasty give good functional outcome, while also minimizing morbidities that are usually associated the use of a custom-made prosthesis or complex arthroplasty.
Clinical Message
In endemic regions, hydatid disease of the bone must be considered in the differential diagnosis of atypical aggressive infections. Debridement and excision along with appropriate medical anti-helminthic treatment are necessary to treat the disease.
References
1. Beggs I. The radiology of hydatid disease. AJR Am J Roentgenol 1985;145(3):639-648.1. Beggs I. The radiology of hydatid disease. AJR Am J Roentgenol 1985;145(3):639-648.
2. Gossios KJ, Kontoyiannis DS, Dascalogiannaki M, Gourtsoyiannis NC. Uncommon locations of hydatid disease: CT appearances. Eur Radiol 1997;7(8):1303-1308.
3. Morar R, Feldman C. Pulmonary echinococcosis. Eur Respir J 2003;21(6):1069-1077.
4. Eckert J, Schantz PM, Gasser RB, Torgerson PR, Bessonov AS, Movsessian SO, et al. Geographic distribution and prevalence. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski Z, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: Office International des Epizooties Paris; 2001. p. 100-142.5. Amir-Jahed AK, Fardin R, Farzad A, Bakshandeh K. Clinical echinococcosis. Ann Surg 1975;182(5):541-546.
6. Ertem M, Karahasanoglu T, Yavuz N, Erguney S. Laparoscopically treated liver hydatid cysts. Arch Surg 2002;137(10):1170-1173.
7. Palanivelu C, Jani K, Malladi V, Senthilkumar R, Rajan PS, Sendhilkumar K, et al. Laparoscopic management of hepatic hydatid disease. JSLS 2006;10(1):56-62.
8. Tekin A, Kücükkartallar T, Kartal A, Kaynak A, Ozer S, Tavli S, et al. Clinical and surgical profile and follow up of patients with liver hydatid cyst from an endemic region. J Gastrointestin Liver Dis 2008;17(1):33-37.
9. Menghebat L, Jiang L, Chai J. A retrospective survey for surgical cases of cystic echinococcosis in the Xinjiang Uygur Autonomous Region, PRC (1951–90). In: Andersen F, Chai J, Liu F, editors. Compendium on Cystic Echinococcosis with Special Reference to the Xinjiang Uygur Autonomous Region, People’s Republic of China. Provo: Brigham Young University Print Services; 1993. p. 135-145.
10. Zlitni M, Ezzaouia K, Lebib H, Karray M, Kooli M, Mestiri M. Hydatid cyst of bone: Diagnosis and treatment. World J Surg 2001;25(1):75-82.
11. Torricelli P, Martinelli C, Biagini R, Ruggieri P, De Cristofaro R. Radiographic and computed tomographic findings in hydatid disease of bone. Skeletal Radiol. 1990;19(6):435-439.
12. Ozer AF, Ozek MM, Pamir MN, Erzen C. Magnetic resonance imaging in the diagnosis of spinal hydatid cyst disease. Case report. Paraplegia 1993;31(5):338-340.
13. Tekkök IH, Benli K. Primary spinal extradural hydatid disease: Report of a case with magnetic resonance characteristics and pathological correlation. Neurosurgery 1993;33(2):320-323.
14. Song XH, Ding LW. Bone hydatid disease. Postgraduate Medical Journal. 2007;83(982):536-542.
15. Belzunegui J, Maíz O, López L, Plazaola I, González C, Figueroa M. Hydatid disease of bone with adjacent joint involvement. A radiological follow-up of 12 years. Br J Rheumatol 1997;36(1):133-135.
16. Agarwal S, Shah A, Kadhi SK, Rooney RJ. Hydatid bone disease of the pelvis. A report of two cases and review of the literature. Clin Orthop Relat Res 1992;280:251-255.
17. Siwach R, Singh R, Kadian VK, Singh Z, Jain M, Madan H, et al. Extensive hydatidosis of the femur and pelvis with pathological fracture: A case report. Int J Infect Dis 2009;13(6):e480-e482.
18. Steinmetz S, Racloz G, Stern R, Dominguez D, Al-Mayahi M, Schibler M, Lew D, Hoffmeyer P, Uçkay I. Treatment challenges associated with bone echinococcosis. J Antimicrob Chemother. 2014;69(3):821-826.
19. Wirbel RJ, Mues PE, Mutschler WE, Salomon-Looijen M. Hydatid disease of the pelvis and the femur. A case report. Acta Orthop Scand 1995;66(5):440-442.
20. Natarajan MV, Kumar AK, Sivaseelam A, Iyakutty P, Raja M, Rajagopal TS. Using a custom mega prosthesis to treat hydatidosis of bone: A report of 3 cases. J Orthop Surg (Hong Kong) 2002;10(2):203-205.
21. Nath P, Bhattacharya S, Dutta V, Joshi GR, Patel M. Primary Iliac Bone Hydatid Disease: An Unusual Presentation. Med J Armed Forces India 2009;65(2):180-181.
22. Neelapala VS, Chandrasekar CR, Grimer RJ. Revision hip replacement for recurrent Hydatid disease of the pelvis: A case report and review of the literature. J Orthop Surg Res 2010;11(5):17.
23. Notarnicola A, Panella A, Moretti L, Solarino G, Moretti B. Hip joint hydatidosis after prosthesis replacement. Int J Infect Dis 2010;14 Suppl 3:e287-e290.
 |
 |
 |
 |
 |
| Dr. Nishit Bhatnagar | Dr. Hari Kishan | Dr. Sumit Sural | Dr. Purushotham Lingaiah | Dr. Kameshwaran Jaikumar |
| How to Cite This Article: Bhatnagar N, Kishan H, S Sural, Lingaiah P, Jaikumar K. Pelvic Hydatid Disease: A Case Report and Review of Literature. Journal of Orthopaedic Case Reports 2017 Jul-Aug;7(4):25-28 |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




