[box type=”bio”] Learning Point of the Article: [/box]
Osteomyelitis may arise from nosocomial infections. Special attention should be paid to the presence of organisms uncommonly seen in community-acquired infections, particularly when detected after operative intervention.
Case Report | Volume 9 | Issue 4 | JOCR July-August 2019 | Page 71-75 | Walter Dehority, Selina Silva, Martha Muller. DOI: 10.13107/jocr.2019.v09i04.1486
Authors: Walter Dehority[1], Selina Silva[1], Martha Muller[1]
[1]Department of Pediatrics, The University of New Mexico School of Medicine, Albuquerque, New Mexico.
Address of Correspondence:
Dr. Walter Dehority,
Department of Pediatrics, The University of New Mexico School of Medicine,MSC10 5590, 1 University of New Mexico, Albuquerque 87131-0001, New Mexico.
E-mail: wdehority@salud.unm.edu
Abstract
Introduction: Pseudomonas osteomyelitis in otherwise healthy children is rare but may present following puncture wounds to the foot or involve the skull following mastoiditis. We present a case of nosocomial chronic osteomyelitis of the tibia caused by Pseudomonas in a previously healthy adolescent following surgical debridement of a non-pseudomonal chronic osteomyelitis in the same location 18 months prior. To our knowledge, such a case has not previously been described.
Case Report: A 17-year-old previously healthy young man presented with several month duration of pain in the right leg below the knee with no prior trauma, overlying a site of chronic, culture-negative osteomyelitis which was successfully treated with anti-staphylococcal therapy 1year prior. Magnetic resonance imaging of the affected area revealed findings consistent with chronic osteomyelitis at his prior surgical site, while operative culture demonstrated growth of a multidrug-resistant Pseudomonas aeruginosa. An extensive immunological evaluation was unremarkable. He demonstrated clinical and radiographic improvement following 4 months of intravenous antimicrobial therapy.
Conclusion: Given the extreme rarity of P.aeruginosa in community-acquired osteoarticular infections, as well as the antimicrobial resistance profile demonstrated and the localization to a prior surgical site, this is felt to be the first description of a nosocomial osteoarticular infection with this organism in an otherwise healthy child. Providers should be aware of the potential for osteoarticular infection with atypical organisms in the post-operative patient.
Keywords: Chronic osteomyelitis, nosocomial, Pseudomonas.
Introduction
Nosocomial osteomyelitis is uncommon in children, particularly nosocomial chronic osteomyelitis [1, 2]. Such infections are typically confined to post-operative infections following placement of orthopedic implants and often in children with numerous medical comorbidities [3]. Osteomyelitis associated with Pseudomonas aeruginosa is also rarely described in children and is most often reported following complications of localized infections with the organism, such as mastoiditis, or in post-traumatic or implant-associated infections [3, 4]. We describe the first case of a nosocomial chronic osteomyelitis due to P.aeruginosa in an otherwise healthy adolescent.
Case Report
A 16-year-old previously healthy young man presented to the Pediatric Infectious Disease Clinic in September of 20151week after arriving in the United States from a refugee camp in Mozambique with a 3-month history of pain and swelling in the upper leg just below the knee. He had no fever noted, no erythema over the knee, and his symptoms had not changed appreciably since arrival in the United States. He had not received any antimicrobial therapy for this condition. His erythrocyte sedimentation rate (ESR) at presentation was 40 mm/h with a C-reactive protein (CRP) level of 0.9 g/dL and a leukocyte count of 6,900 cells/µL (50% polymorphonuclear cells, 37% lymphocytes, 11% monocytes, 1% eosinophils, and 1% basophils). On examination, his affected knee was slightly swollen with mild tenderness over the medial aspect. No discharge or erythema was noted. His range of motion for his knee was slightly decreased for flexion and extension, and an antalgic gait was present. At that time, a plain radiograph demonstrated concerns of chronic osteomyelitis of the right proximal tibia although tumor or subacute fracture was also considered (Fig. 1). Contrasted magnetic resonance imaging (MRI)demonstrated findings consistent with chronic osteomyelitis along the medial aspect of the proximal tibial epiphysis and metaphysis, however, with a cloaca extending from a cortical disruption of the posteromedial tibial metaphyseal cortex (Fig. 2).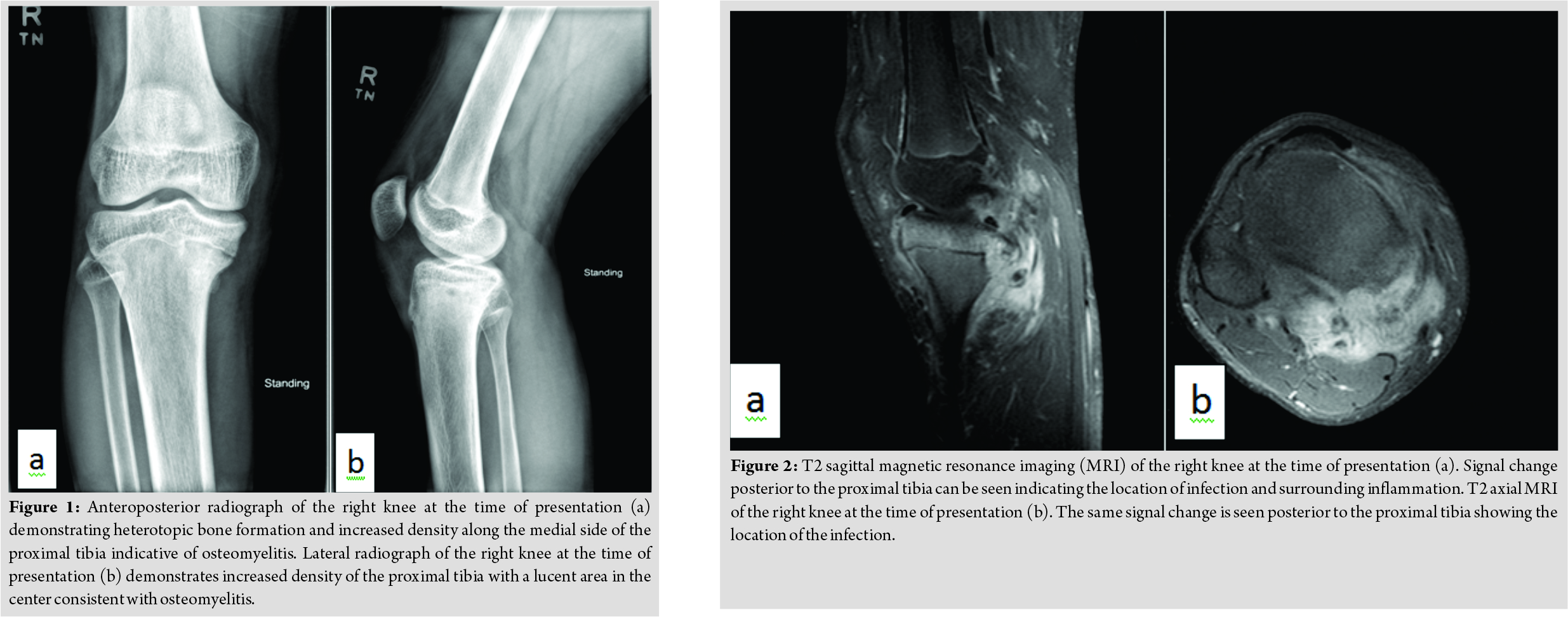 Given a desire to pursue potential debridement and obtain cultures simultaneously, he underwent surgical debridement of the area the following day, cultures from which were sterile. Given his recent exposure to a refugee camp in Africa, the resulting potential for infection with an unusual organism was also felt to justify the need for an open biopsy with cultures. A cortical window was not created although extensive debridement of the area was performed. The joint space was not entered during the surgery. No orthopedic hardware or antimicrobial-impregnated beads were placed. Post-operatively, his wound healed well, with resolution of a serous drainage after several days. Given a non-reactive QuantiFERON gold assay for Mycobacterium tuberculosis and the frequency with which Staphylococcus aureus is implicated in osteoarticular infections in otherwise healthy children, we pursued empiric therapy for methicillin-susceptible S. aureus with nafcillin (chosed for its potent activity against this pathogen and ability to administer as a continuous infusion at home). He demonstrated marked improvement after completing 6 weeks of parenteral therapy, and his ESR and CRP normalized approximately 8 weeks into treatment. Per our local practice guidelines, he completed 6 months of total therapy with cephalexin in April of 2016. Given involvement of the growth plate with his infection, follow-up imaging was obtained 10 months post-operatively, which revealed a 1.3 cm leg length discrepancy (right leg shorter than his left). He was seen again in the infectious disease clinic 3 months after antibiotic completion and was back to his normal baseline, with plain radiographs demonstrating no concerns of recrudescent disease. A follow-up MRI was not obtained. He played a season of varsity soccer for his local high school that fall without incident.
Given a desire to pursue potential debridement and obtain cultures simultaneously, he underwent surgical debridement of the area the following day, cultures from which were sterile. Given his recent exposure to a refugee camp in Africa, the resulting potential for infection with an unusual organism was also felt to justify the need for an open biopsy with cultures. A cortical window was not created although extensive debridement of the area was performed. The joint space was not entered during the surgery. No orthopedic hardware or antimicrobial-impregnated beads were placed. Post-operatively, his wound healed well, with resolution of a serous drainage after several days. Given a non-reactive QuantiFERON gold assay for Mycobacterium tuberculosis and the frequency with which Staphylococcus aureus is implicated in osteoarticular infections in otherwise healthy children, we pursued empiric therapy for methicillin-susceptible S. aureus with nafcillin (chosed for its potent activity against this pathogen and ability to administer as a continuous infusion at home). He demonstrated marked improvement after completing 6 weeks of parenteral therapy, and his ESR and CRP normalized approximately 8 weeks into treatment. Per our local practice guidelines, he completed 6 months of total therapy with cephalexin in April of 2016. Given involvement of the growth plate with his infection, follow-up imaging was obtained 10 months post-operatively, which revealed a 1.3 cm leg length discrepancy (right leg shorter than his left). He was seen again in the infectious disease clinic 3 months after antibiotic completion and was back to his normal baseline, with plain radiographs demonstrating no concerns of recrudescent disease. A follow-up MRI was not obtained. He played a season of varsity soccer for his local high school that fall without incident.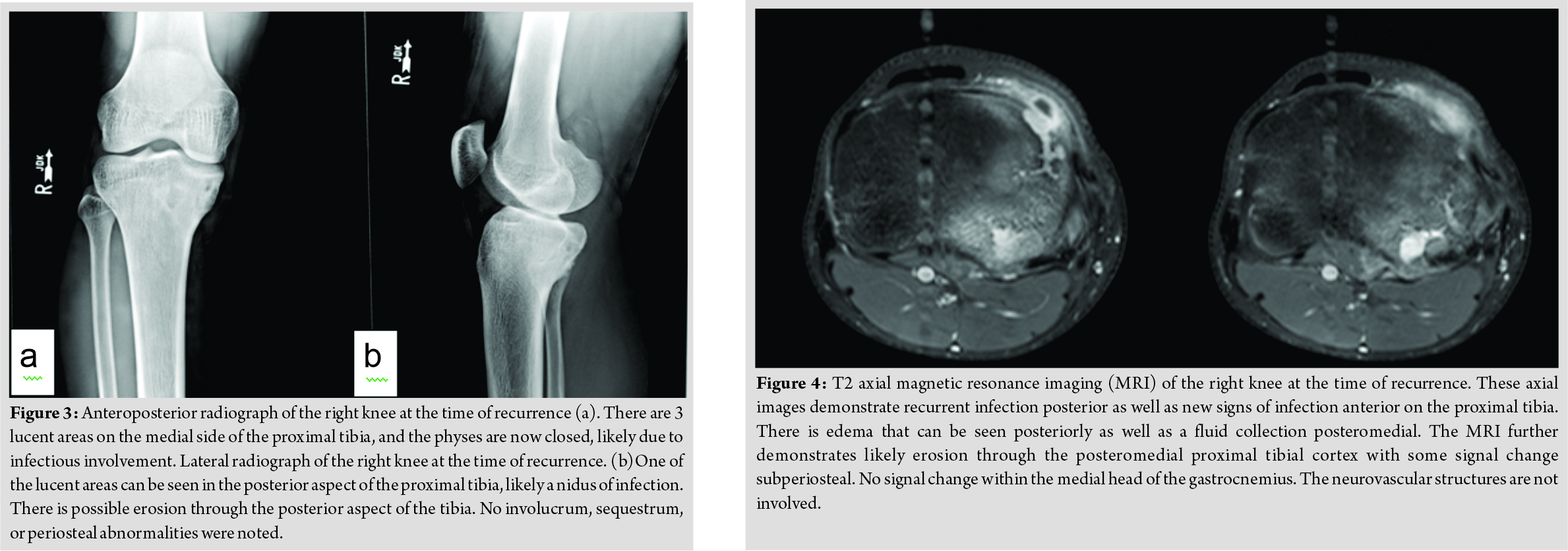
In April of 2017, approximately 1 year after completing therapy, he was seen in the infectious disease clinic again. At that time, the patient reported a 7-day history of pain, redness, and swelling along the inner aspect of the right upper leg below his knee overlying his prior surgical site. However, his father reported that he had complained of these findings for several months. A firm, tender erythematous mass over the medial aspect of the proximal tibia was noted on examination, underlying his surgical scar, although no drainage was present and the range of motion of his knee was normal. He had been a febrile and denied any recent trauma to the area. His ESR and CRP were normal at this time, as was his total leukocyte count and his differential leukocyte count. Plain radiographs demonstrated concern of recrudescent chronic osteomyelitis in the proximal tibia, with worsening sclerotic changes compared with his most recent radiograph (Fig. 3). A contrasted MRI demonstrated abnormal signal intensity in the proximal tibial metaphysis and epiphysis, with an abscess connected to a nidus of presumed chronic osteomyelitis througha fistula tract running inferior to the physis (Fig. 4 and 5). He was taken to the operating room shortly after this, where an abscess eroding through to the posterior aspect of the proximal tibia was noted. The superficial portion of the abscess was drained, with drilling through the affected bone into the posterior aspect of the tibia followed by curettage of the entire area. A cortical window was not created nor was periosteal elevation noted. His post-operative wound healing was uneventful. Operative cultures produced monomicrobial growth of P.aeruginosa, which, given the heavy growth in the primary streaks on the agar plate, was not felt to be a contaminant. This isolate demonstrated intermediate resistance to piperacillin-tazobactam (minimum inhibitory concentration (MIC)=32/4 mg/L), resistance to gentamicin (MIC>2 mg/L), aztreonam (MIC>16 mg/L), and ciprofloxacin (MIC>2 mg/L).The isolate was susceptible to meropenem (MIC of 1 mg/L), cefepime (MIC of 8 mg/L), ceftolozane and tazobactam (MIC=1.5 mg/L), and ceftazidime (MIC of 4 mg/L).Cefepime and ceftazidime MICs were confirmed with E-testing. Noimprovement was seen after 72 h of empiric cefazolin, but he did improve following transition to meropenem. Immunological evaluation revealed normal levels of natural killer cells, immunoglobulins (IgG, IgA, IgM, and IgE), tetanus, and pneumococcal antibodies. A lymphocyte subset panel was normal. He also demonstrated a normal lymphocyte proliferation response to phytohemagglutinin and pokeweed mitogen and a non-reactive serology to human immunodeficiency virus.Given the absence of an oral treatment option, he was treated initially with several days of cefepime, followed by 49 days of meropenem. He was then transitioned back to cefepime for 14 days followed by 56 days of ceftazidime. A contrasted MRI performed 7 weeks and plain radiographs performed 3 months into treatment demonstrated no recrudescence of disease (Fig. 6). He maintained normal inflammatory markers during treatment. At the end of 4 months of therapy, his physical examination was normal and his antibiotic therapy was discontinued. He denied any persistent symptomatology and was participating in soccer again without difficulty.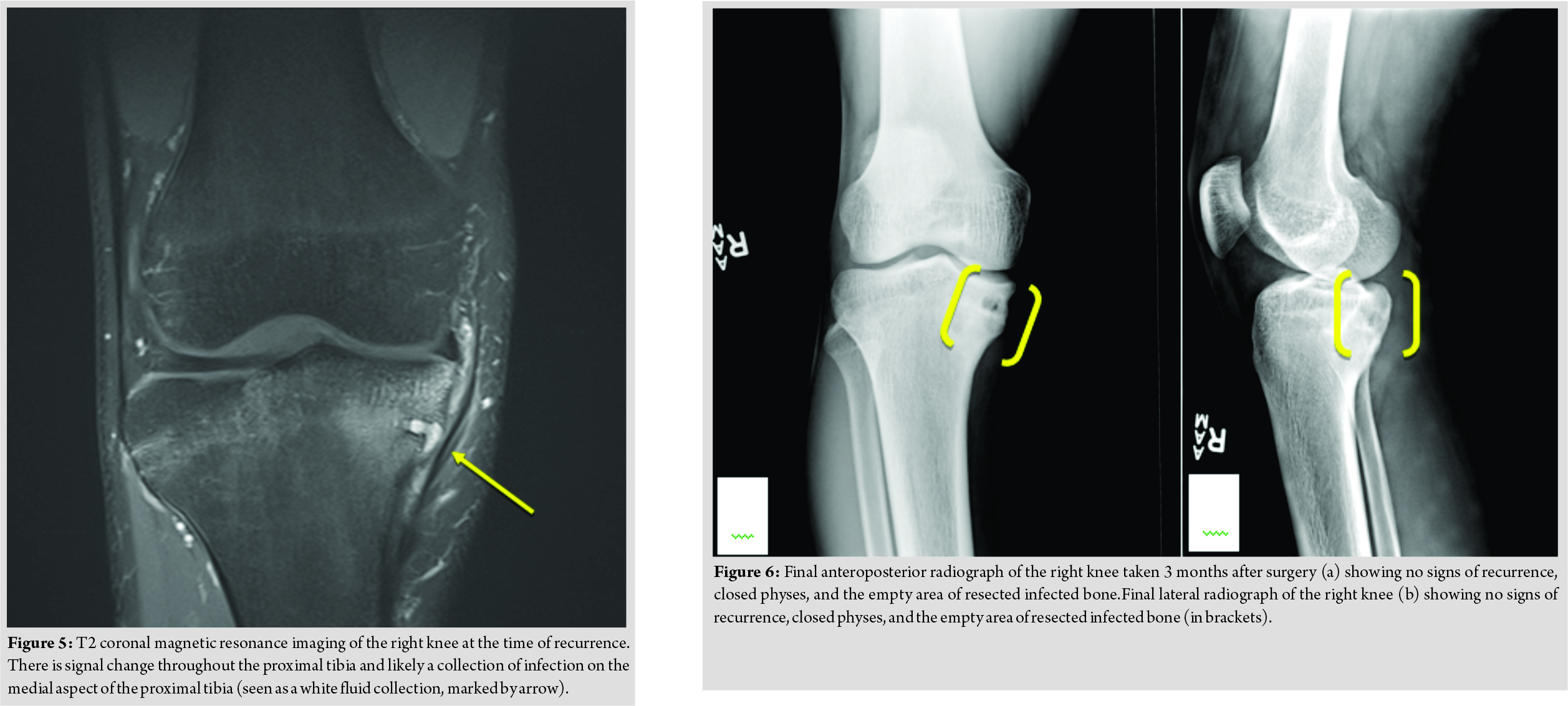
Discussion
Pseudomonas osteomyelitis is rare in otherwise healthy children but may arise as a result of breaches in bone integrity or overlying soft tissues in contact with moist, colonized skin (e.g.,osteomyelitis of the skull arising from mastoiditis or osteomyelitis in the bones of the foot secondary to adjacent puncture wounds) [4, 5]. However, isolated cases from other locations have rarely been reported in otherwise healthy children. Hatakenaka described a 15-month-old previously healthy boy with septic arthritis of the elbow and acute osteomyelitis of the capitellum due to P. aeruginosa following trauma [6]. Narang reported a 13-year-old previously healthy girl with chronic Pseudomonas osteomyelitis of the talus in the absence of reported trauma, while Yousef described chronic osteomyelitis of the clavicle in an otherwise healthy child [7, 8]. Pseudomonas will also occasionally be reported in large case series of pediatric osteomyelitis, usually comprising a very small percentage of the total pathogen burden and without accompanying descriptive clinical data [9]. Although reports of nosocomial osteoarticular infections may be increasing, Pseudomonas is rarely reported and almost exclusively follows placement of orthopedic implants [1, 2, 3]. To our knowledge, this is the first case thought to have arisen from a nosocomial infection in an otherwise healthy child with no orthopedic implants. Approximately 1 year after completing therapy for his first episode of chronic osteomyelitis, our patient presented with recrudescent signs and symptoms in the same location as his prior chronic osteomyelitis. Given the good clinical response seen with nafcillin and cephalexin during treatment for his first episode of osteomyelitis, it is unlikely that this initial infection was due to Pseudomonas. The heavy monomicrobial growth seen in the primary streaks on hisoperative culture, as well as the lack of clinical response seen with his initial empiric cefazolin argue against the organism acting as a contaminant. The results of his immunological evaluation as well as his otherwise good health before his osteomyelitis argue against the infection arising as a result of an occult immunodeficiency. The multidrug resistance profile of the organism was also more in keeping with a nosocomial source [10]. Finally, the recurrence at his prior surgical site also argued for a role as a nosocomial pathogen. The timing of the infection is unclear; however, it may have been introduced at the time of his initial surgery, lying quiescent as an indolent infection that ultimately became symptomatic. However, he presented with his second episode of osteomyelitis nearly 18 months after this operation. Chronic osteomyelitis with prolonged and indolent courses due to Pseudomonas has been describe although not with latency periods of 18 months [7, 8]. However, the exact timing of his illness was unclear, as his father stated that he had been symptomatic for several months at the time of presentation, which contrasted with what was reported by the patient. Hence, the latency period may actually have been in a more typical range for such an infection. Alternatively, the organism may have colonized his skin following his hospitalization and gained access to the operative site through minor cutaneous trauma and/or transient bacteremia sometime following discharge. With either scenario, the organism most likely arose from a nosocomial source. The absence of an oral option for therapy also complicated his treatment and required the use of a prolonged parenteral regimen. Although an increasing number of multidrug-resistant Gram-negative organisms are appreciated in community-acquired infections in children, such infections are still generally unexpected [11]. The presence of resistance to aminoglycosides, fluoroquinolones, and piperacillin-tazobactam is unusual in a community-acquired isolate and is more often seen in hospital (particularly intensive care) settings, likely due to the accumulation of multiple different resistance mechanisms over time [12]. Based on antimicrobial susceptibility testing data from ourchildren’s hospital for the year 2015, when the patient initially presented for care, 2.9% of all pseudomonal isolates were resistant to gentamicin, 11.4% were resistant to ciprofloxacin, and 14.3% were resistant to piperacillin-tazobactam. Of note, he did not spend any time in the intensive care unit during either of his admissions. Concern did exist regarding the potential for hispseudomonal isolate to develop resistance while on therapy, given the high MICs demonstrated for many of the antimicrobial agents (particularly cefepime), the potential for suboptimal concentrations of antibiotic in the bone, and the proclivity of Pseudomonas to develop resistance while on prolonged therapy [13]. This in part led to our decision to rotate his antibiotics, as well as a desire to avoid prolonged therapy with the broad coverage provided by meropenem, with its attendant risk of adverse effects. However, he demonstrated consistent clinical and radiographic improvement while on therapy. Given the difficulty treating such infections, however, a focus should be kept on prevention. Appropriate antimicrobial stewardship interventions in hospitals are imperative to decrease the burden of such multidrug-resistant organisms in the inpatient setting, as is meticulous attention to sterile technique intraoperatively and wound care post-operatively.
Conclusion
We believe that this is the first case of a nosocomial osteomyelitis in an otherwise healthy child due to P. aeruginosa in the literature. Although rare, when faced with children presenting with concerns of recrudescent or recurrent osteomyelitis, clinicians should consider the possibility of nosocomial infections with multidrug-resistant organisms acquired while hospitalized.
Clinical Message
Although rare, recurrent osteomyelitis at the previous site of disease should raise a suspicion of a nosocomial infection. As such, culture of the affected area is imperative to help identify and guide treatment of potentially multidrug-resistant organisms.
References
1. Chaves MS, Franco D, Nanni JC, Basaldúa ML, Boleas M, Aphalo G, et al. Control of an outbreak of postoperative bone mucormycosis: An intervention study of contiguous cohorts. Am J Infect Control 2016;44:1715-7.
2. Pigrau C, Rodríguez-Pardo D, Fernández-Hidalgo N, Moretó L, Pellise F, Larrosa MN, et al. Health care associated hematogenous pyogenic vertebral osteomyelitis: A severe and potentially preventable infectious disease. Medicine (Baltimore) 2015;94:e365-85.
3. Messina AF, Berman DM, Ghazarian SR, Patel R, Neustadt J, Hahn G, et al. The management and outcome of spinal implant-related infections in pediatric patients: Aretrospective review. Pediatr Infect Dis J 2014;33:720-3.
4. Amir AZ, Pomp R, Amir J. Changes in acute mastoiditis in a single pediatric tertiary medical center: Our experience during 2008-2009 compared with data for 1983-2007. Scand J Infect Dis 2014;46:9-13.
5. Laughlin TJ, Armstrong DG, Caporusso J, Lavery LA. Soft tissue and bone infections from puncture wounds in children. West J Med 1997;166:126-8.
6. Hatakenaka T, Uemura K, Itsubo T, Hayashi M, Uchiyama S, Kato H, et al. Septic arthritis of the elbow in a child due to Pseudomonas aeruginosa: A case report. J PediatrOrthop B 2014;23:285-7.
7. Narang S. Pseudomonas osteomyelitis of the talus: Review of the pathophysiology and report of a rare case. J Foot Ankle Surg 2009;48:672-6.
8. Yousef A, Pace A, Livesley P. Chronic haematogenousPseudomonas aeruginosa osteomyelitis of the clavicle, a case report and review of the literature. Eur J Pediatr2006;165:424-6.
9. Saavedra-Lozano J, Mejías A, Ahmad N, Peromingo E, Ardura MI, Guillen S, et al. Changing trends in acute osteomyelitis in children: Impact of methicillin-resistant Staphylococcus aureus infections. J PediatrOrthop2008;28:569-75.
10. Geisinger E, Isberg RR. Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J Infect Dis 2017;215:S9-17.
11. Moxon CA, Paulus S. Beta-lactamases in Enterobacteriaceae infections in children. J Infect 2016;72 Suppl:S41-9.
12. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009;22:582-610.
13. Ang JY, Abdel-Haq N, Zhu F, Thabit AK, Nicolau DP, Satlin MJ, et al. Multidrug-resistant Pseudomonas aeruginosa infection in a child with cystic fibrosis. Antimicrob Agents Chemother 2016;60:5627-30.
 |
 |
 |
| Dr. Walter Dehority | Dr. Selina Silva | Dr. Martha Muller |
| How to Cite This Article: Dehority W, Silva S, Muller M. Nosocomial Chronic Osteomyelitis of the Tibia in an Otherwise Healthy Adolescent: A Case Report. Journal of Orthopaedic Case Reports 2019 Jul-Aug; 9(4): 71-75. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




