[box type=”bio”] What to Learn from this Article?[/box]
Vacuum-assisted closure system should be used in post-operative infections after spinal instrumented surgeries.
Case Report | Volume 7 | Issue 1 | JOCR January – February 2017 | Page 95-100| Maya Kale, Pravin Padalkar, Varshil Mehta. DOI: 10.13107/jocr.2250-0685.706
Authors: Maya Kale[1], Pravin Padalkar[2], Varshil Mehta[3]
[1] Department of Microbiology, MGM Medical College, Navi Mumbai, Maharashtra, India,
[2] Center for Orthopaedic & Spine surgery, 105 , Neel Enclave, Sec 09, Khanda Colony, New Panvel, India.
[3] Department of Internal Medicine, MGM Medical College, Navi Mumbai, Maharashtra, India.
Address of Correspondence
Dr. Varshil Mehta,
103, Sky High Tower, Orlem, Tank Road, Malad West, Mumbai – 400 064, Maharashtra, India.
E-mail: varshil91@gmail.com
Abstract
Introduction: Post-operative wound infections after spinal surgery is a very serious problem, leading to a risk of significant morbidity which may even lead to prolonged hospitalization. Various treatment protocols have been recommended for debridement, antibiotic, and soft-tissue management, but with mixed results. However, the risk of morbidity is still high with these treatment options. Vacuum-assisted closure (VAC) system has been gaining popularity recently in the management of subacute, acute, and chronic wounds. This study aims to review the use of the indigenous VAC in the management of deep infections after spinal instrumentation surgery.
Case Series: Between 2010 and 2015, 12 out of 514 patients who developed a deep infection after spinal surgery, were selected and reviewed retrospectively at multiple centers (MGM Hospital, Kamothe and Center for Orthopaedic & Spine Surgery, New Panvel, Navi Mumbai, India). Out of 12 patients, one of the patients needed a partial implant exchange although none of the cases needed complete implant removal. All patients had achieved clean closed wounds along with a retention of the instrumentation. There was no need for flap surgery to cover wound defect in any case. However, antibiotic treatment was necessary in all cases. None of the patients showed a new infection after the treatment.
Conclusion: The study demonstrates the usefulness of VAC therapy as an alternative management for wound conditioning of a back wound with the high complexity in nature after instrumented spine surgeries as it eliminates complex secondary surgeries, prolong use of antibiotics and removal of the implants.
Keywords: Spinal infection, wound closure, vacuum-assisted closure.
Introduction
Post-operative wound infections after spinal surgery is a very serious problem, leading to a risk of significant morbidity which may even lead to prolonged hospitalization. The rate of post-operative infections after spine surgery observed in literature is between 0.4% and 20% [1, 2, 3, 4, 5]. Furthermore, this rate is found to be increased with an increase in complexity of the performed procedure [6, 7, 8]. Till date, many treatment protocols have been recommended for debridement, antibiotic, and soft-tissue management, but with mixed results [9, 10, 11, 12]. However, the risk of morbidity is still high with these treatment options. With that said, the use of the wound vacuum-assisted closure (VAC) procedure (Triage Meditake) has been gaining popularity recently, in the management of subacute, acute, and chronic wounds (Fig. 1) [13, 14, 15, 16, 17]. The controlled application of subatmospheric pressure dressing (SPD)in the VAC, not only helps the formation of granulation tissue but also, assists in the debridement of necrotic tissue and acts as a sterile barrier. Increasing usage of the VAC procedure for the complex soft tissue injuries has shown an accelerated wound healing as compared to that of traditional methods [16, 18, 19, 20]. This study aims to review the use of the indigenous VAC system in the management of deep infection after spinal instrumentation surgery. It retrospectively analyzes a series of deep subfascial infection after instrumented spine surgeries operated over a period of 5 years which have been managed by VAC. This is primarily based on the concept of temporary tissue coverage and reduction of dead space and secondary closure of the wound. It demonstrates the usefulness of VAC therapy as alternative management for wound conditioning of back wound after instrumented spine surgeries. It eliminates the need of implant removals and helps to salvage instrumented spine. It may also avoid the necessity for complex plastic surgeries and reoperation later.
Case Series
Materials and methods
Between May 2010 and 2015, spinal surgeries were performed in 514 patients at our institution. The ethical approval was done from the Institutional Ethics Committee. Indications for surgery were spine fractures, spinal fusion procedures for spondylolisthesis in degenerative diseases, instrumentation for Koch’s spine, and sagittal and coronal spinal deformity correction procedures.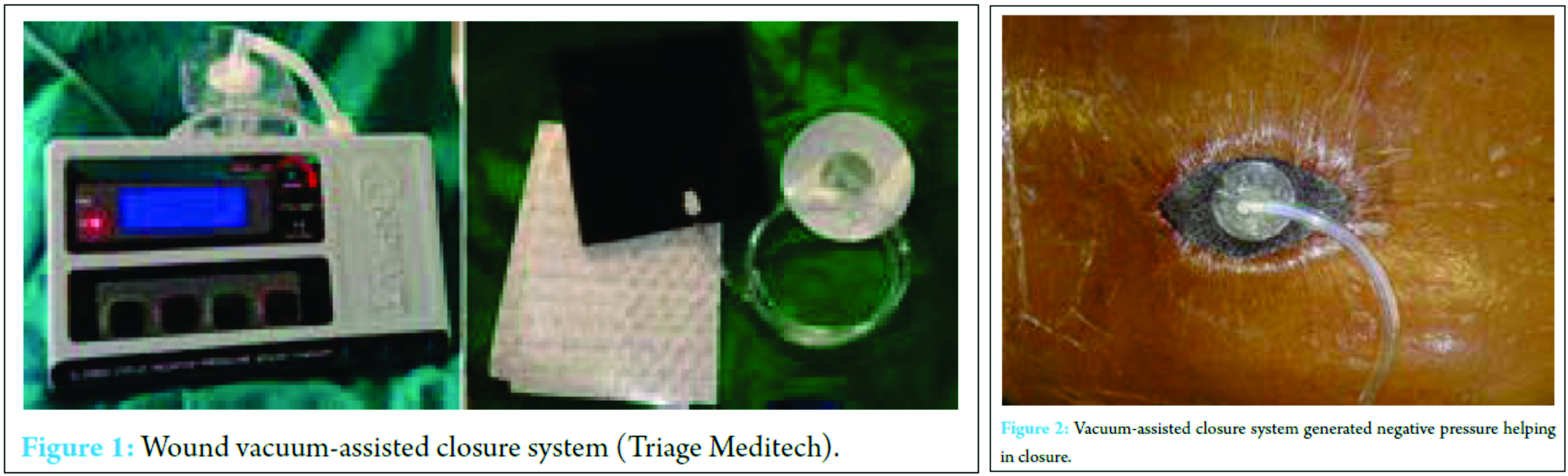
A total of 12 patients developed a deep infection (2.3%) and were reviewed retrospectively in this study. The physical and medical notes, history of previous surgeries, pre-operative admission history, risk factors, and comorbidities of all 12 patients were recorded. The time interval between the surgery and the occurrence of infection was noted. In addition, the other data – such as duration of the surgery, the operative surgical and anesthetic reports, estimated blood loss and transfusion number, and pre-operative antibiotic prophylactics which were given – were also noted. The infection was monitored throughout by microbiological analysis of the etiological organism and by the number of debridement. Average duration of the post-operative antibiotic treatment and the time required for secondary closure from the day of VAC application was also recorded. All cases of VAC applications were done under aseptic condition in operation theater. Decision was made to close wound secondarily after adequate granulation tissue formation on wound. Delayed suture removal was done at an interval of 3 weeks.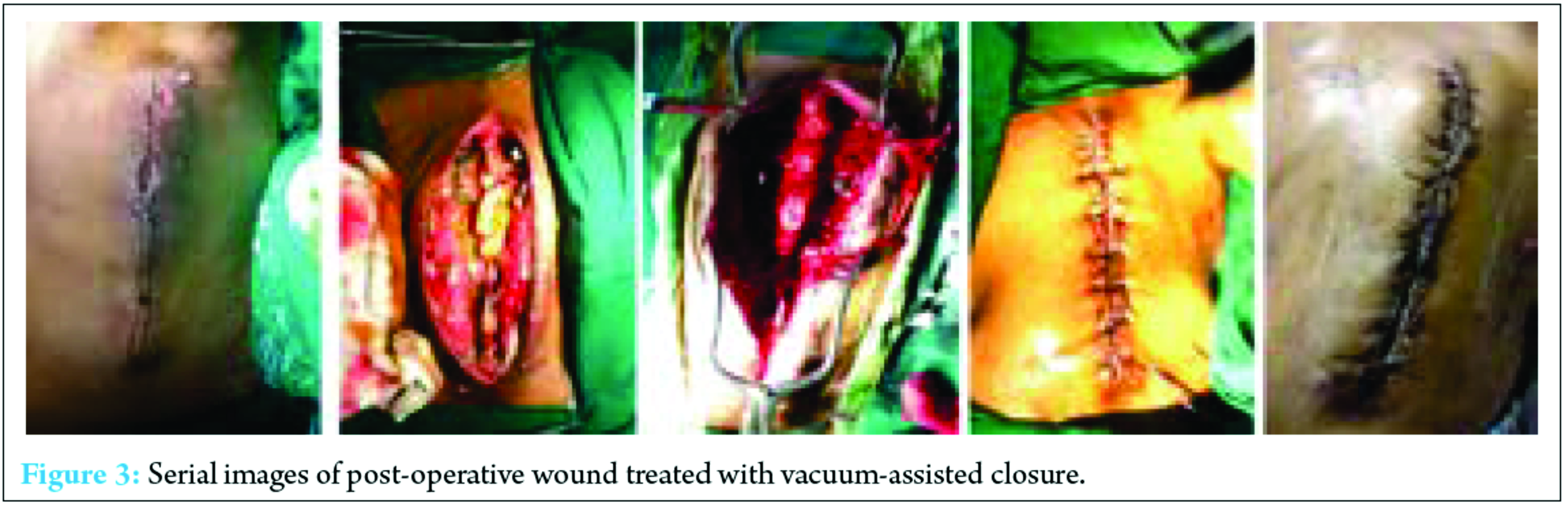
All patients were received intravenous antibiotics (amikacin 750 mg and cefuroxime 1.5 g) after indexed surgery as prophylactic antibiotics. Antibiotic therapy was continued without changing to different doses or drugs until culture and sensitivity reports were obtained from intraoperative samples. Specific antibiotic therapy was then given based on reports of culture and sensitivity. The decision to use the VAC system was done by the surgeon (author) on basis of the underlying disease and the macroscopic appearance of the wound. The VAC system includes black polyurethane soft foam which is cut to fit the cavity of the wound and then placed to fill the entire wound area, i.e., dead space in various layers if necessary. A transparent adhesive fluid- and gas-impermeable plastic film is pasted over the foam and about of the wound surroundings to make a wound seal. A hole is then cut in the center, and a specific designed adhesive TRAC-PAD is fixed over it. The latter is then attached to a suction tube through a container with a suction pump which is adjustable. A negative pressure of 125 mmHg is continuously generated which leads to a uniform negative pressure all over the collapsed foam which brings the wound fluid into the foam and the container from the wound (Fig. 1 and 2) [21].
Results
Of the 514 patients, 12 were patients treated for surgical site infections suggesting an incidence rate as 2.3%. The mean age of the infected patients was 48 years (range 18-75 years). There were 5 females and 7 males. Other factors associated with increased risk of infection were chronic renal failure, malnutrition, smoking, diabetes, rheumatoid arthritis, and alcohol abuse were also recorded. Indication for posterior spinal instrumentation and nature of operation performed is listed in Table 1. Deep drains were used in the primary procedure in all cases and removed about 48 h postoperatively. The average surgery time was 2.5 (range 1.5-3.25) h. The infection presented on a mean of 15 days after surgery. The average time of the previous spinal surgery to first revision surgery was 12 (range 7-18) days, and VAC duration of patients was 12.16 (range 5-24) days. The mean follow-up of the infected patients was 15 (range 12-18) months. One of the cases needed a partial implant exchange although none of the cases needed complete implant removal. All patients achieved clean closed wounds with retention of the instrumentation. There was no need for flap surgery to cover wound defect in any case. Antibiotic treatment was necessary in all cases. The mean duration of parenteral and oral antibiotic therapy was 1.83 (range 1-7) weeks and 3 (range 2-6) weeks, respectively. Normalization of laboratory markers were observed at an average of 4.2 (range 3-6) weeks. Average two cycles (14 days) of VAC therapy were required before secondary closure. A mean of 2.1 (range 3-8) debridement and irrigation procedures were conducted before the wound closure operation as per definition. None of the patients showed a new infection after treatment. The mean follow-up period was 13 (range 12-16) months.
Discussion
VAC is a technique which helps in healing of infected wounds resistant to treatment by established methods. It is been widely used in infected and post-operative wounds. As stated above, VAC has been gaining popularity in the management of acute, subacute, and chronic wounds. It act as a sterile barrier and prevents further contamination of wound and its negative suction pressure helps in debridement of the necrotic tissue and enhance neovascularization [20]. Blum et al. reviewed 229 open tibial fractures with 72% receiving negative pressure wound therapy (NPWT) and 28% conventional dressings retrospectively. They found a significantly reduced deep infection rate in the NPWT group (8.4% vs. 20.6%, P = 0.01) [22]. In another study conducted by Sinha et al. in which random 30 open musculoskeletal injuries were either subjected to NPWT dressings changed every 3-4 days or standard dressings daily. While dressings were changed, measurements were taken each time, and at day 4 and 8 post-initial debridement, tissue biopsies were done for histopathological analysis. They concluded a significantly reduced wound size in the NPWT group over the period of 8 days (mean 13.24 mm vs. 3.02 mm, P = 0.0001), significantly increased angiogenesis, granulation tissue and fibrosis (Wilcoxon signed-rank test P < 0.05), and a reduction in bacterial growth by day 8 (60% no growth vs. 20%). All patients healed without infection, one required a free flap [23]. Hence, the backbone of VAC is its negative pressure. However, the heart of the VAC system is a microprocessor-controlled vacuum unit that is capable of providing controlled levels of either intermittent or continuous SPD ranging from 25 to 200 mmHg with 125 mmHg as being generally used [24]. VAC is also know by many other names such as topical negative pressure, SPD, vacuum sealing technique, and sealed surface wound suction [25]. VAC can be used in almost any type of wounds including pressure ulcers, acute/trauma wounds, diabetic wounds, burns, dehisced surgical wounds, leg ulcers, rotational/free flaps, and post-surgical infectious wounds [26]. Post-operative infections after instrumented spine surgery have been reviewed previously in literature in terms of occurrence rate, microbiology, complications, and surgical technique [27, 28, 29]. The risk factors which compromise local perfusion and thus leading to an infection are smoking, diabetes, alcohol abuse, morbid obesity, immune deficiency in the case of malignancy, cardiovascular problems, and radiation before surgery [12]. The flap coverage is yet a standard treatment of infected wounds after instrumental spine surgery [30, 31, 32]. However, flap closure is accompanied with significant morbidity, blood loss, including extended operative time, recurrent infection, seroma, dehiscence, flap failure, donor site morbidity, significant comorbidities, and poor tissue characteristics which can complicate the wound healing or even compromise the chosen flap [33, 34, 35]. Recently, various studies have been conducted which concludes that successful management of post-operative infection after spinal instrumentation surgery without flap coverage is possible [9, 12, 36, 37]. Such treatment includes repetitive debridement, antibiotic medications, delayed closure, local irrigation system, and maintenance of the instrumentation system. Nevertheless, this method was considered as inappropriate in one of the study in which 13 of 19 patients developed wound complications, chronic infection, wound dehiscence, and hematoma [38]. Debridement without replacement or removal of the implant combined with prolonged intravenous and oral antibiotic treatment has a failure rate between 32% and 86% [39]. Deep infection which is persistent, often necessitates the removal of hardware [40, 41]. One of the study has observed that the removal of hardware rates in patients with deep spinal infection was up to 35% [40]. Richards et al. in his study had removed the hardware in all of his 10 patients with deep infections after scoliosis surgery [42]. Fig. 3 depicts serial images of post-operative wounds treated with VAC. In reality, the main purpose of any method is to manage the infections appropriately and shorten the duration of hospitalization with a reduction in the need of antibiotics intake and the implantation removal. This study showed that VAC therapy has been successful in preventing the need of long-term antibiotic therapy and removal of implants as compared to the old methods.
Conclusion
The study demonstrates the usefulness of VAC therapy as alternative management for wound conditioning of back wound with the high complexity in nature after instrumental spine surgeries. It eliminates need of implant removal and help to salvage instrumented spine. There was no need for long-term antibiotic therapy and use of higher antibiotic was prevented when therapy was used in conjugation with VAC therapy. The use of the VAC system is specifically appealing in patients with two or more comorbidities since it may not only avoid the need for complex plastic surgery but also, reoperation later.
Clinical Message
This study shows us the advantages of using VAC in patients with post-operative infections after spinal instrumented surgery. It helps to close the wound, prevents the prolongation of morbidity and antibiotics usage and implants removal. Hence, VAC should be used in all post-operative infections after spinal instrumented surgeries.
References
1. Malamo-Lada H, Zarkotou O, Nikolaides N, Kanellopoulou M, Demetriades D. Wound infections following posterior spinal instrumentation for paralytic scoliosis. Clin Microbiol Infect 1999;5(3):135-139.
2. Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000;25(19):2461-2466.
3. Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: A review of 2,391 consecutive index procedures. Clin Spine Surg 2000;13(5):422-426.
4. Wendt JR, Gardner VO, White JI. Treatment of complex postoperative lumbosacral wounds in nonparalyzed patients. Plast Reconstr Surg 1998;101(5):1248-1253.
5. Wimmer C, Gluch H. Management of postoperative wound infection in posterior spinal fusion with instrumentation. J Spinal Disord 1996;9(6):505-508.
6. Faraj AA, Webb JK. Spinal instrumentation for primary pyogenic infection report of 31 patients. Acta Orthop Belg 2000;66(3):242-247.
7. Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res 1992;284:99-108.
8. Roberts FJ, Walsh A, Wing P, Dvorak M, Schweigel J. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine (Phila Pa 1976) 1998;23(3):366-370.
9. Dernbach PD, Gomez H, Hahn J. Primary closure of infected spinal wounds. Neurosurgery 1990;26(4):707-709.
10. Perry JW, Montgomerie JZ, Swank S, Gilmore DS, Maeder K. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis 1997;24(4):558-561.
11. Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine (Phila Pa 1976) 2001;26(18):1990-1996.
12. Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumented lumbar fusions complicated by surgical wound infection. Spine 1996;21(18):2163-2169.
13. Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, et al. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg 2000;66(12):1136-1143.
14. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96(9):488-492.
15. Greer S, Kasabian A, Thorne C, Borud L, Sims CD, Hsu M. The use of a subatmospheric pressure dressing to salvage a Gustilo grade IIIB open tibial fracture with concomitant osteomyelitis to avert a free flap. Ann Plast Surg 1998;41(6):687.
16. Mehbod AA, Ogilvie JW, Pinto MR, Schwender JD, Transfeldt EE, Wood KB, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech 2005;18(1):14-17.
17. Yuan-Innes MJ, Temple CL, Lacey MS. Vacuum-assisted wound closure: a new approach to spinal wounds with exposed hardware. Spine (Phila Pa 1976) 2001;26(3):E30-E33.
18. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38(6):563-576.
19. Mooney JF 3rd, Argenta LC, Marks MW, Morykwas MJ, DeFranzo AJ. Treatment of soft tissue defects in pediatric patients using the VAC (TM) system. Clin Orthop Relat Res 2000;376:26-31.
20. Singh K, Samartzis D, Heller JG, An HS, Vaccaro AR. The management of complex soft-tissue defects after spinal instrumentation. J Bone Joint Surg Br 2006;88(1):8-15.
21. Labler L, Keel M, Trentz O, Heinzelmann M. Wound conditioning by vacuum assisted closure (V.A.C.) In postoperative infections after dorsal spine surgery. Eur Spine J 2006;15(9):1388-1396.
22. Blum ML, Esser M, Richardson M, Paul E, Rosenfeldt FL. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma 2012;26(9):499-505.
23. Sinha K, Chauhan VD, Maheshwari R, Chauhan N, Rajan M, Agrawal A. Vacuum assisted closure therapy versus standard wound therapy for open musculoskeletal injuries. Adv Orthop 2013;2013:245940.
24. Thomas S. An introduction to the use of vacum assisted closure. World Wide Wounds; 2001. Available from: http://www.worldwidewounds.com/2001/may/Thomas/Vacuum-Assisted-Closure.html. [Last accessed on 2016 Dec 31].
25. Banwell PE, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12(1):22-28.
26. Nursing Times, Vacuum-assisted Closure. Vol. 97. p. 51. Available from: https://www.nursingtimes.net/clinical-archive/wound-care/vacuum-assisted-closure/200663.article. [Last accessed on 2016 Dec 31].
27. Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord 1997;10(4):317-324.
28. Davne SH, Myers DL. Complications of lumbar spinal fusion
| How to Cite This Article: Kale M, Padalkar P, Mehta V. Vacuum-Assisted Closure in Patients with Post-operative Infections after Instrumented Spine Surgery: A Series of 12 Cases. Journal of Orthopaedic Case Reports 2017 Jan-Feb;7(1):95-100. Available from: https://www.jocr.co.in/wp/wp-content/uploads/27.-2250-0685.706.pdf |
Authors Gallery |
||
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com







