 [box type=”bio”] Learning Point of the Article: [/box]
[box type=”bio”] Learning Point of the Article: [/box]
Pairing nerve hydrodissection with active tendon and nerve gliding exercises in patients with carpal tunnel syndrome receiving platelet-rich plasma injections may produce outcomes comparable to surgical release.
Case Report | Volume 10 | Issue 9 | JOCR December 2020 | Page 38-46 | Nathan J Savage, Joseph Albano. DOI: 10.13107/jocr.2020.v10.i09.1896
Authors: Nathan J Savage[1], Joseph Albano[2]
[1]Department of Physical Therapy, Total Rehab, Inc., South Ogden, UT, USA.
[2]Albano Clinic, Salt Lake City, UT, USA.
Address of Correspondence:
Dr. Nathan J Savage,
Department of Physical Therapy , 5957 South Fashion Point Drive, Suite 102, South Ogden, UT 84315.
E-mail: nathan@totalrehabclinics.com
Abstract
Introduction: Hydrodissection has been used during injection procedures to liberate median nerve from surrounding adhesions. This investigation examined clinical and neurophysiologic impact of ultrasound-guided injections in patient with bilateral carpal tunnel syndrome (CTS) serving as own control. Novel to this investigation was performance of active tendon and nerve gliding exercises following median nerve hydrodissection and injection.
Case Report: A 37-year-old male with 6-year history of bilateral CTS presented for treatment. Wrists randomly assigned to receive platelet-rich plasma (PRP) or equal volume saline injection and median nerve hydrodissection. The patient performed active tendon and nerve gliding exercises following injection procedures. Pain ratings, CTS-related disability scores, median nerve function, and median nerve cross-section area measurements for each wrist/hand collected at baseline 2, 4, 6, and 12 months following injection procedures. 6-month follow-up. The right (saline) and left (PRP) wrists showed improvements in disability and nerve function. The left wrist (PRP) also showed improvement in pain. 1-year follow-up. The right (saline followed by PRP at 6 months) and left (PRP) wrists showed improvements in pain, disability, and nerve function.
Conclusion: Results suggest innovative treatment approach for CTS, namely, ultrasound-guided PRP injection including median nerve hydrodissection followed by performance of active tendon and nerve gliding exercises in immediate post-injection period. The patient demonstrated improvements in pain, CTS-related disability, and median nerve function comparable to surgical release and generally better than non-surgical interventions. Findings should stimulate further investigation into marrying mechanically based treatments with PRP to produce better long-term outcomes in patients with CTS.
Keywords: Electrodiagnosis, median neuropathy, physical therapy, ultrasonography.
Introduction
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy in the body [1, 2, 3, 4, 5] with an overall prevalence in the general population of 5.3% for women and 2.1% for men with certain occupations experiencing higher incidence [6, 7]. Patients with CTS complain of pain and paresthesia in the hand (often worse at night) that typically follows a median nerve distribution although symptom location varies and can include non-median innervated fingers or radiate proximally into the forearm or arm [4, 5, 8]. CTS is diagnosed based on patient history and physical examination findings [4, 5] with electrodiagnostic (EDX) testing [1, 2, 9, 10] and neuromuscular ultrasonography (NMUS) [11, 12, 13, 14, 15] routinely performed for the purposes of differential diagnoses and quantification of median nerve function and morphology. EDX testing, including neurography and needle electromyography (EMG), provides reliable and valid measures of median nerve function in patients with suspected CTS and can be used to classify severity of median neuropathy when present [1, 2, 10]. Point-of-care NMUS allows for dynamic imaging of volar wrist structures including median nerve morphology and cross-sectional area (CSA) in addition to adjacent flexor tendons, vasculature, and connective tissue [11, 12, 14, 15, 16, 17]. Treatment of CTS varies depending on symptom severity, symptom duration, and patient preference [3, 8, 18, 19]. Carpal tunnel release surgery is the most common wrist/hand surgery performed in the USA with nearly 500 K annually and a total economic cost exceeding $2 billion [3, 18]. Non-surgical treatment approaches include physical therapy, splinting, NSAIDs, oral steroids, and injection procedures [8, 19, 20, 21, 22, 23]. Physical therapy often employs active tendon and nerve gliding exercises [20, 24], which are mechanically based treatments seeking to stimulate soft-tissue healing and improve median nerve vascularization within the carpal tunnel [25, 26]. Tendon and nerve gliding exercises can decrease edema, improve median nerve mobility by reducing adherence of surrounding connective tissue, and decrease nociception by decreasing concentrations of pro-inflammatory substances and reduce peripheral and central nervous system sensitization [3, 20, 22, 27]. In patients with CTS, tendon and nerve gliding exercises may improve short- to mid-term outcomes when combined with other treatments [7]. A variety of injection procedures has been to treat CTS. Steroid injections are common and provide significant short- to mid-term symptomatic relief in patients with mild-moderate disease but have not been shown to provide long-term resolution [21, 23, 28, 29, 30, 31, 32] and likely have a negative impact on soft tissue and nerve health overtime [23]. Given the limited long-term effectiveness of steroid injections for treating CTS, recent investigations have examined a variety of “biologic” compounds including platelet-rich plasma (PRP) as a more suitable injectate because of the demonstrated neuroregenerative and tissue healing effects [17, 31, 33, 34, 35, 36, 37, 38]. PRP is a safe and natural alternative to surgery for treating a variety of soft-tissue injuries including neuropathy [35, 36, 37, 38]. Rich in growth factors and cytokines with anti-inflammatory effects, PRP promotes removal of degenerative and necrotic tissues and may enhance soft-tissue healing and regeneration [34]. The potential for PRP to provide permanent tissue healing and regeneration has stimulated research into its use for a number of conditions including CTS [31, 34, 35, 36, 38]. Recent studies have demonstrated the beneficial healing effects of PRP on a variety of human tissues including peripheral neuropathies like CTS [16, 30, 31, 32, 39, 40, 41, 42]. However, variation exists with regard to volume of injectate used, injection approach (e.g., palmer vs. ulnar), and whether or not median nerve hydrodissection was performed [31, 43, 44, 45, 46]. Debate exists over the value of performing median nerve hydrodissection during an injection procedure for treating CTS [43]. Several studies have demonstrated that in patients with CTS, the sub-synovial connective tissue surrounding the median nerve and adjacent tendons is thickened and fibrotic decreasing median nerve mobility [25, 45, 47, 48]. This decreased mobility has been hypothesized to exacerbate median neuropathy by making it more susceptible to injury. Hydrodissection is used to liberate the median nerve from surrounding connective tissue adhesions, thereby improving mobility, increasing vascularization, and stimulating axoplasmic transport for improved nerve health [43, 44, 45, 46]. Wu et al. [45] demonstrated the therapeutic value of median nerve hydrodissection as a stand-alone treatment in patients with CTS but it remains unclear if hydrodissection provides additional benefit over injection alone [43]. This triple-blind case report examined the clinical and neurophysiologic impact of US-guided injections, comparing saline and PRP, in a patient with EDX confirmed bilateral CTS serving as their own control. Novel to this investigation was the addition of active tendon and nerve gliding exercises to median nerve hydrodissection and injection.
Case Report
Methods
Patient characteristics
Subject: A 37-year-old male; BMI 27.7 (Height = 1.98 meters, weight = 109 kg); right-handed; self-employed cable worker (i.e., heavy manual labor); reported daily tobacco and alcohol use. The patient reported 6-year history of CTS worse on right. Prior treatments included night splints and chiropractic 94 care. The patient denied prior injection or surgery to either wrist/hand. Following clinical and NMUS examinations, the patient consented to undergo EDX testing, which confirmed bilateral CTS and excluded other neuromuscular conditions. The patient consented to study participation and was offered PRP injection in wrist that received saline if PRP proved more effective at 6-month follow-up.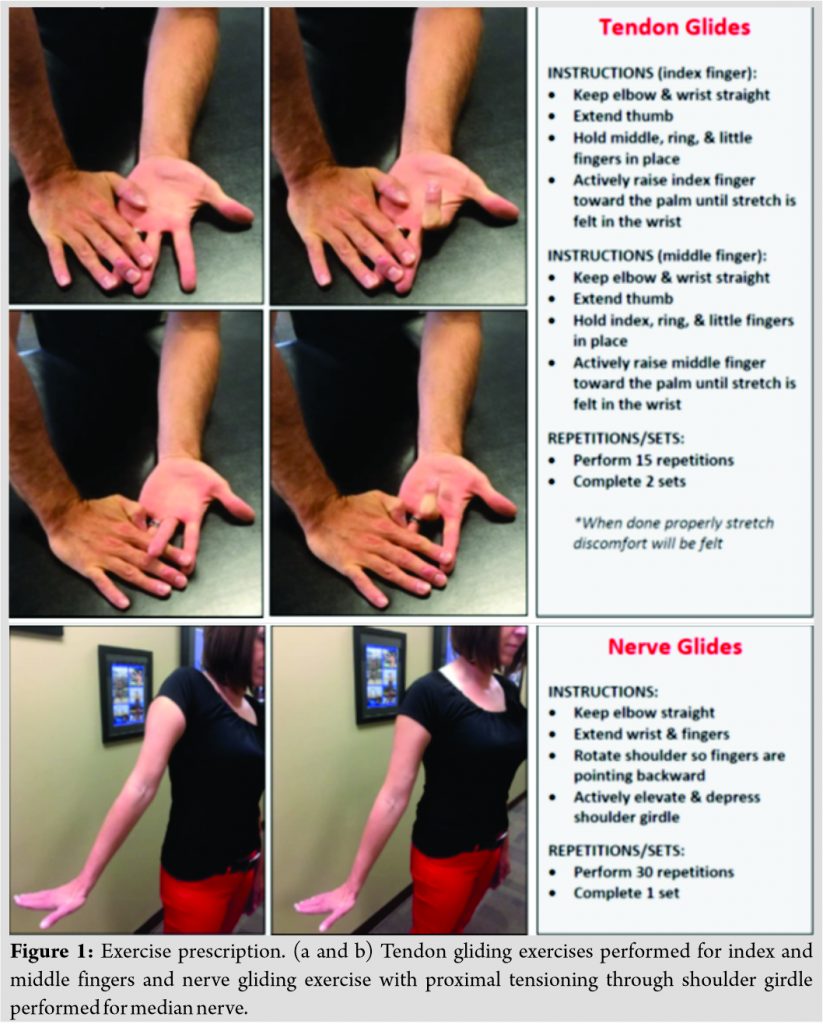
Study design and procedures
The study conducted in private outpatient orthopedic and sports medicine clinic from early April 2018 to late March 2019. The patient provided written informed consent before baseline data collection, which included numeric pain rating scale (NPRS) and Boston carpal tunnel questionnaire (BCTQ) scores for each wrist/hand along with neurography and median nerve CSA measurements of both wrists/hands. The patient instructed in active tendon and nerve gliding exercises and provided a detailed exercise handout and journal for immediate post-injection period.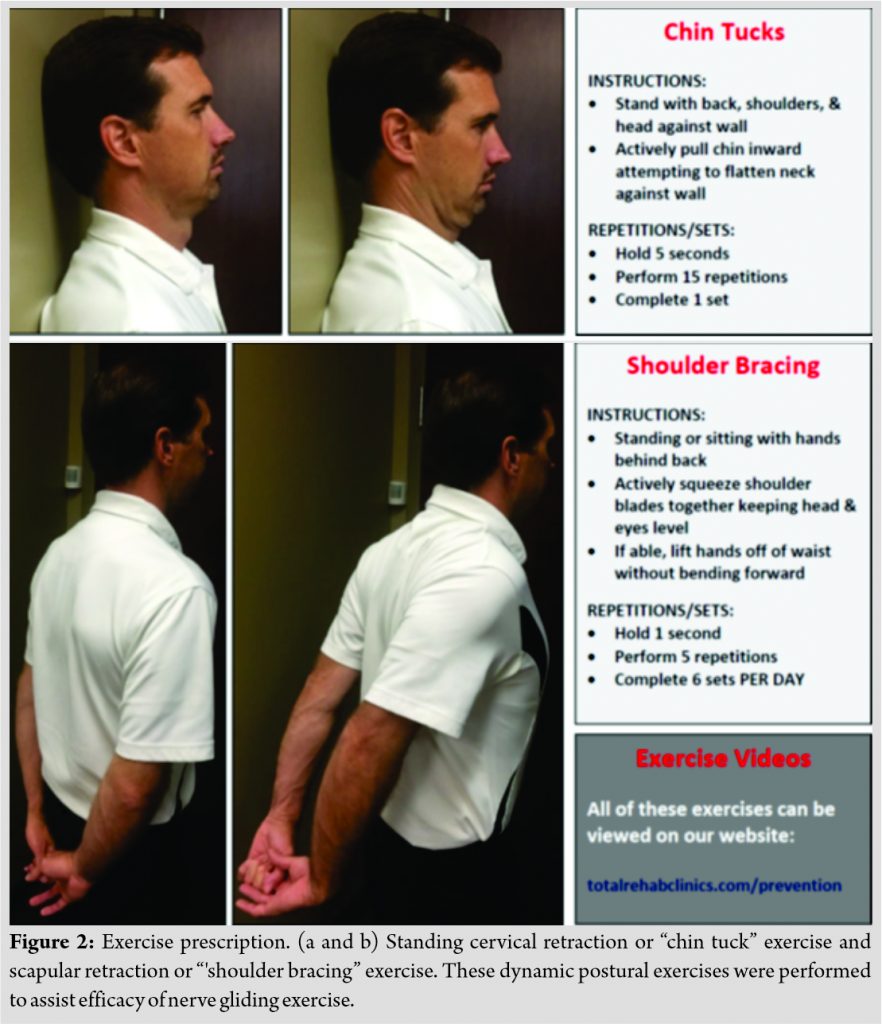
Injections
Blood drawn and PRP prepared. Wrists randomly assigned to receive PRP or equal volume saline and blinded injections prepared. US-guided injections performed including median nerve hydrodissection. Following injections, wrists splinted and the patient provided verbal and written instructions for symptom management, avoidance of NSAIDs, and to begin prescribed exercises when symptoms allow.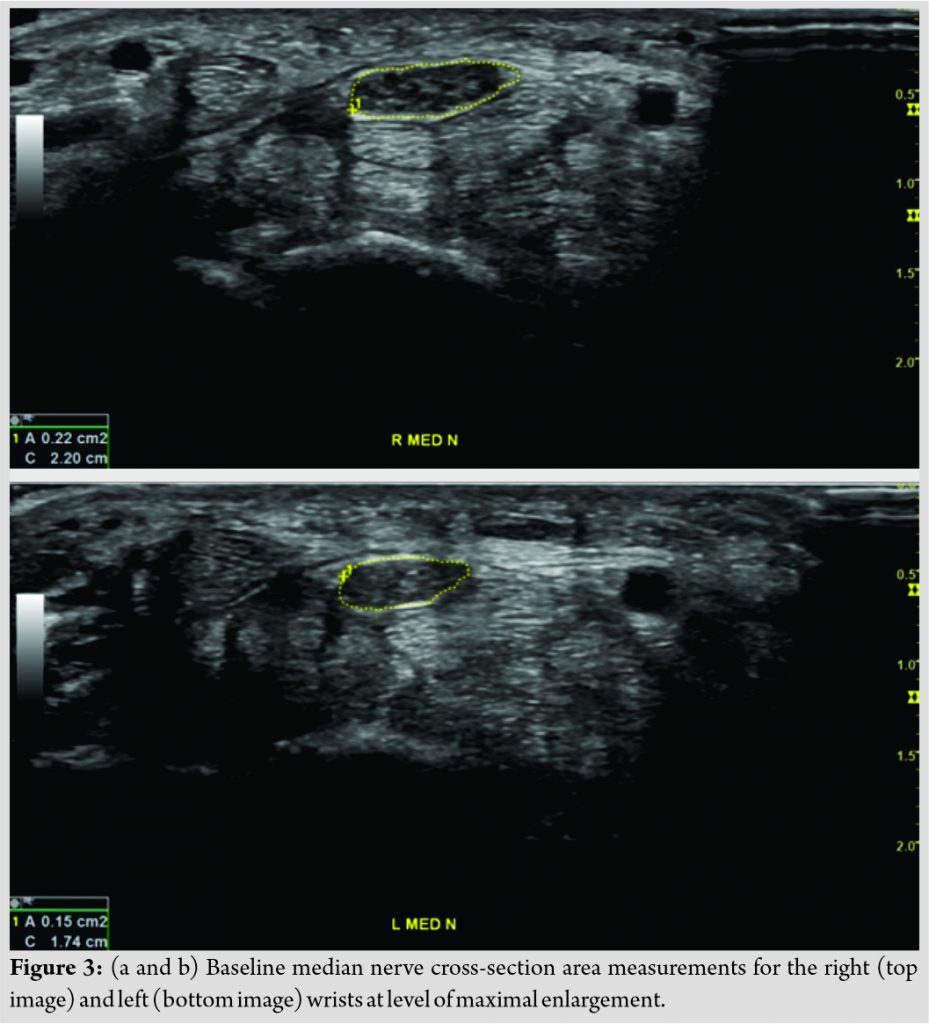
Follow-up visits
Initial follow-up 2 months post-injection patient’s exercise journal collected and exercises discontinued. NPRS and BCTQ scores, neurography, and median nerve CSA measurements performed for each wrist/hand at 2, 4, and 6-month follow-up visits. Following data collection at 6-month follow-up wrists unblinded and injectate revealed. As previously discussed, if wrist that received PRP showed more improvement than wrist that received saline patient could elect to have PRP injection and encouraged to follow-up 6 months later (1 year since beginning of study). The patient would be prescribed same post-injection tendon and nerve gliding exercises.
Patient-reported outcomes
NPRS
Eleven-point pain scale with scores ranging from 0 “no pain” to 10 “worst imaginable pain” for current, best, and worst levels of pain over previous 24 h. Minimal clinically important difference (MCID) for NPRS is ~2.0 points [49]. Separate ratings collected for each wrist/hand.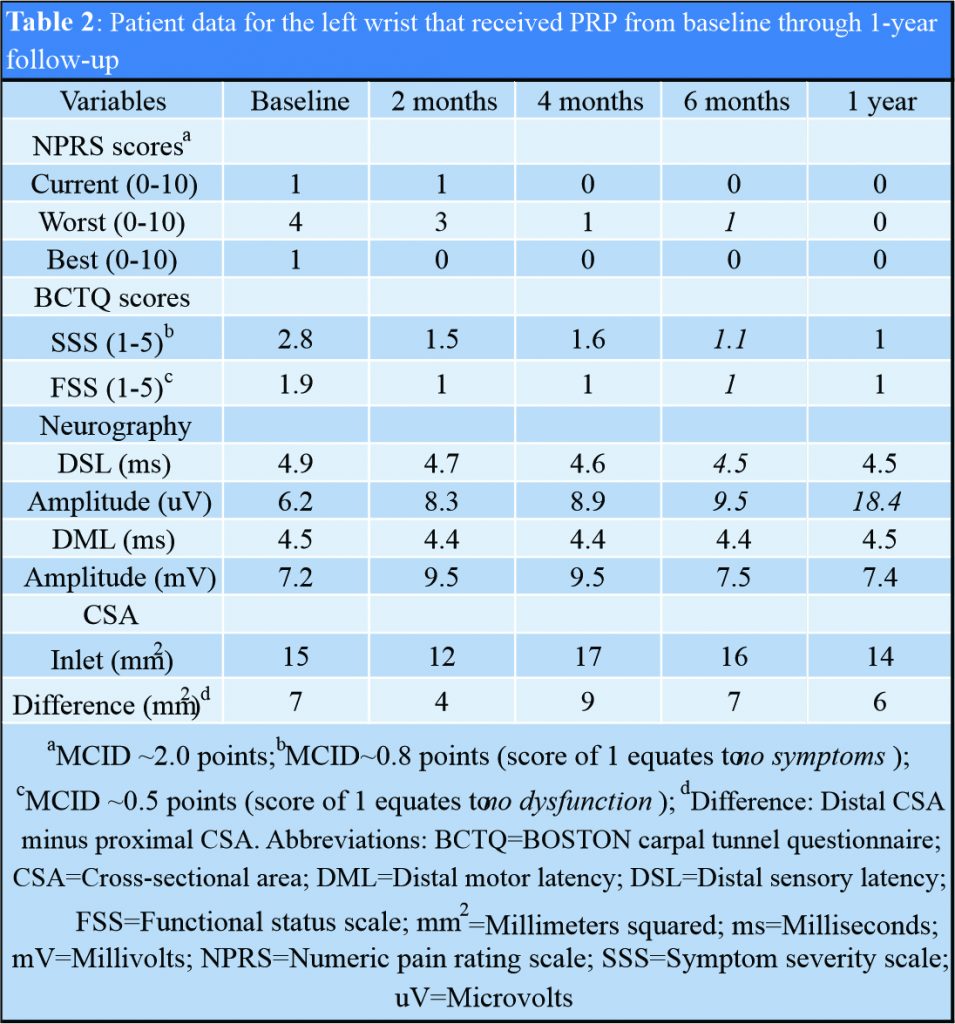
BCTQ
CTS-related disability questionnaire consisting of an 11-question Symptom Severity Scale (SSS) and an 8-question Functional Status Scale (FSS). Scores in both scales range from 1 to 5 points with higher scores equating to more 120 severe symptoms or higher levels of dysfunction and a value of 1 equating to the absence of symptoms or dysfunction. BCTQ has been shown to have good reproducibility, internal consistency, and validity in patients with CTS [50, 51, 52]. MCID for SSS is ~0.8 points and for FSS ~0.5 points [53, 54].
Ultrasonography
NMUS was conducted by a regenerative medicine physician with 30 years of clinical experience and who is registered in Musculoskeletal® sonography by the Alliance for Physician Certification and Advancement and was blinded to neurography findings and injection assignment throughout study. NMUS performed using GE Logiq P9 system (GE Healthcare, Chicago, IL, USA) with 12–15 MHz linear transducer. Short- and long-axis images obtained in each wrist and digitally stored including flexor retinaculum, flexor tendons, median nerve, carpal joints, and ulnar nerve in Guyon’s canal. Short-axis images stored of median nerve proximally at level of pronator quadratus muscle and distally at level of maximum enlargement for calculation of CSA using direct trace method inside echogenic epineurium [55]. For the purposes of classification, increase in median nerve CSA of >2 mm2 comparing distal to proximal values considered diagnostic of CTS with 99% accuracy [15]. In addition, increase of median nerve CSA of ≥6 mm2 suggests “moderate” disease and ≥9 mm2 suggests “severe” disease.
Electrodiagnosis
EDX testing was conducted by a physical therapist with 20 years of clinical experience and who is Board Certified in Clinical Electrophysiology by the American Board of Physical Therapy Specialties and was blinded to NMUS findings and injection assignment throughout study. EDX testing was performed using Cadwell Sierra Wave system (Cadwell Laboratories, Kennewick, WA, USA). The patient underwent standardized peripheral sensory and motor neurography of median and ulnar nerves including F waves [9]. Monopolar needle EMG was performed in both upper extremities analyzing resting and volitional muscle activity representing C5-T1 nerve roots. For the purposes of classification, prolonged distal sensory latency (DSL) only considered “mild” CTS; prolonged DSL and distal motor latency (DML) considered “moderate” CTS; and significantly prolonged or absent DSL and DML and abnormal EMG activity in the abductor pollicis brevis muscle considered “severe” CTS.
Injection procedures
PRP preparation
Thirty milliliters (mL) whole blood drawn from antecubital vein using ENDORET® Technology (BTI Biotechnology Institute, Blue Bell, PA, USA) yielding 8.0 mL of plasma-rich growth factors containing 2–3 times baseline concentration of platelets with low red and white blood cell counts. Calcium chloride (0.02 cc/mL) added to preparation before injection.
US-guided injections
The patient seated with forearms supinated, palms facing up, and wrists extended over towel roll. Using in-plane approach, median nerve identified at inlet of proximal carpal tunnel at a level of pisiform bone [55] and ulnar artery identified using Doppler imaging. A 25-gauge needle passed from ulnar side of wrist toward median nerve. Each wrist received 8 mL total injection volume and underwent median nerve hydrodissection from superficial flexor retinaculum and underlying sub-synovial connective tissue. Median nerve in the right wrist adhered more strongly to surrounding soft tissues and required 6 mL of injectate to achieve hydrodissection, whereas the left wrist median nerve required 3 mL of injectate to achieve hydrodissection. Following injection, carpal tunnel visualized in long-axis ensuring proximal-to-distal diffusion of injectate occurred. The patient observed post-procedure for 20 min and provided verbal and written instructions to avoid use of NSAIDs, apply ice for 10–15 min hourly as needed for pain relief, and begin prescribed exercises when symptoms allow.
Exercise prescription
The patient provided verbal and written instructions along with printed illustrations for performance of the following exercises in each wrist/hand: Index and middle finger tendon gliding and median nerve gliding (Fig. 1); cervical retraction and scapular retraction (Fig. 2). The patient instructed to perform exercises until 2-month follow-up visit and was provided an exercise journal to record compliance.
Results
Baseline diagnostic testing suggested right median nerve more involved than left being classified as “moderate-severe” compared with “moderate” CTS, respectively (Fig. 3). At 6-month follow-up, it was revealed that the right wrist received saline and left wrist PRP. Results for pain ratings, CTS-related disability, neurography, and CSA measurements from baseline through 1-year presented in (Table 1, 2) for the right and left wrists, respectively. Note that the right wrist, which initially received saline, subsequently received PRP approximately 1 month (Nov 2, 2018) following the 6-month follow-up visit (October 2, 2018).
6-month follow-up
The right wrist (saline) showed significant improvements in FSS score along with appearance of previously absent median sensory response and improved median DML and amplitude beginning at 2 months (Table 1). The left wrist (PRP) showed significant improvements in worst pain beginning at 4 months, improvements in SSS and FSS scores beginning at 2 months, and modest improvements in median DSL and amplitude beginning at 2 months (Table 2).
1-year follow-up
The right wrist (saline, PRP at 6 months) showed significant improvement in worst pain along with continued improvements in SSS and FSS scores. Previously absent median sensory response appearing 2 months following saline injection remained unchanged in DSL but showed 112% increase in amplitude. Median DML gradually improved overtime beginning 2 months after saline with amplitude relatively unchanged following PRP (Table 1). The left wrist (PRP) showed persistence of previously observed improvements or continued improvements in worst pain, SSS, and FSS scores. Median DSL maintained previously observed improvement in contrast to amplitude that increased 94% compared to 6-month follow-up (Table 2). No significant changes observed in median nerve CSA in either wrist throughout study. The patient denied taking additional medications or receiving additional therapies beyond those prescribed throughout study. No adverse side effects or nerve injury observed or reported for either wrist throughout study.
Discussion
This triple-blind case report examined the clinical and neurophysiologic impact of US-guided carpal tunnel injections, comparing saline, and PRP, in a patient with EDX confirmed bilateral CTS serving as their own control over a 1-year follow-up period. This is the first investigation marrying active tendon and nerve gliding exercises to median nerve hydrodissection in the immediate post-injection period regardless of injectate. We theorized that combining mechanically based treatments would result in better long-term outcomes by promoting median nerve healing and regeneration regardless of injectate. Furthermore, we theorized that combining mechanically based treatments with PRP injection would further stimulate median nerve healing and regeneration by pairing therapeutic movement with an environment rich in growth factors leading to reduced symptoms and improved function [20, 22, 24, 26, 31]. Our hypotheses were borne out in this case report based on the magnitude of improvement in pain, CTS-related disability, and median nerve function which persisted for 1-year. Although injecting PRP was more effective than saline for reducing pain, reducing CTS-related disability, and improving aspects of median nerve function at 6 months, meaningful improvements were observed in the wrist receiving saline for CTS-related disability and median nerve function at 6 months, which may in part be explained by the therapeutic effects performing active tendon and nerve gliding exercises following median nerve hydrodissection. Median nerve hydrodissection has been shown to provide therapeutic benefits independent of injectate. Wu et al. [45], in a randomized trial investigating US-guided median nerve hydrodissection in patients with mild-moderate CTS, demonstrated significant improvements in CTS-related disability and median nerve CSA with modest improvements in median nerve function at 6 months compared to control. The authors highlighted the role that pathological edema and inflammatory-driven synovial thickening play in pathogenesis of CTS by increasing carpal tunnel pressure and reducing median nerve mobility. They concluded that median nerve hydrodissection is a simple, minimally invasive procedure that can be effectively accomplished using saline and provides therapeutic benefit in patients with mild-moderate CTS. Given these results, it would appear that the theoretical rationale has been established for combining median nerve hydrodissection with active tendon and nerve gliding exercises in patients with CTS. To the best of our knowledge, this case report is the first investigation of PRP for treating CTS with a 1-year follow-up. Wu et al. [42], in a single-blind clinical trial compared PRP with night splints in 60 patients with mild-moderate unilateral CTS, demonstrated significant improvements in pain, CTS-related disability, and median nerve CSA at 6 months. Because our study utilized similar methods and outcome measures, comparing our findings with those of Wu et al. is instructive. Both studies performed US-guided median nerve hydrodissection and PRP injection, although our injection volume was larger (8 mL vs. 3 mL, respectively). Baseline pain was higher in their study (6/10 vs. 4/10, respectively) with similar scores observed at 6 months (2/10 and 1/10, respectively). CTS-related disability was similar at baseline for both studies (45 points and 46 points, respectively) with similar scores observed at 6 months (25 points and 20 points, respectively). Median nerve function was similar at baseline in both studies and both studies observed modest but insignificant improvements overtime. In contrast to our study, Wu et al. showed significant improvements in median nerve CSA at 6 months (14 mm2 reduced to 11 mm2) while our study showed a 1 mm2 increase. Overall, our findings were similar at 6 months with observed improvements in pain, CTS-related disability, and median nerve function persisting or improving at 1 year. Shi et al. [56], in a systematic review and meta-analysis, compared short- and long-term outcomes of surgical and non-surgical interventions for treating CTS. For CTS-related disability following surgery, they found an average SSS of 1.1 at 6 months and 1.6 points at 1 year; following non-surgical interventions, these values were 1.9 at 6 months and 1.8 points at 1 year. In our study, SSS for the left wrist (PRP) was 1.1 at 6 months and 1.0 points at 1 year; the right wrist these values were 2.5 points at 6 months (saline) and 1.6 points at 1 year (6 months following PRP). Shi et al. found following surgery and non-surgical interventions, the average FSS was 1.6 points at 6 months and 1.8 points at 1 year. In our study, FSS for the left wrist (PRP) was 1.0 point at 6 months and 1 year; the right wrist these values were 1.4 points at 6 months (saline) and 1.0 point at 1 year (6 months following PRP). Finally, Shi et al. found that the average improvement in median sensory latency at 6 months was −1.1 ms following surgery and −0.5 ms following non-surgical interventions. In our study, improvement in median sensory latency at 6 months for the left wrist (PRP) was −0.4 ms; the right wrist (saline) improved from no response to 5.5 ms at 2 months and remained unchanged at 6 months (no improvement 6 months following PRP). Overall, our results for CTS-related disability were comparable to or better than surgery. Notably, the persistence of our results at 1 year may be associated with the addition of active tendon and nerve gliding exercises following median nerve hydrodissection and PRP.
Conclusion
This triple-blind case report demonstrated that US-guided PRP injection is safe and effective for treating CTS over a 1-year follow-up period. In addition, this investigation suggests an innovative approach for the minimally invasive treatment of CTS, namely, US-guided PRP injection with median nerve hydrodissection combined with active tendon and nerve gliding exercises performed during the immediate post-injection period. Our subject demonstrated improvements in pain, CTS-related disability, and median nerve function comparable to surgical release and generally better than non-surgical interventions [56] despite having generally more severe disease than previously published reports. These findings should stimulate further investigation into marrying mechanically based treatments with PRP to produce better long-term outcomes in patients with CTS. Future research is recommended with sufficient statistical power to investigate the possible 3-way interaction between PRP injection with median nerve hydrodissection paired with performance of active tendon and nerve gliding exercises overtime in patients with CTS.
Clinical Message
This case report suggests an innovative approach for the minimally invasive treatment of CTS, namely, US-guided PRP injection with median nerve hydrodissection combined with active tendon and nerve gliding exercises performed during the immediate post-injection period.
References
1. Werner RA. Electrodiagnostic evaluation of carpal tunnel syndrome and ulnar neuropathies. PM R 2013;5 Suppl 5:S14-21.
2. Jazayeri SM, Ashraf A, Karimian H, Moghari A, Azadeh A. Test-retest reliability of transcarpal sensory NCV method for diagnosis of carpal tunnel syndrome. Ann Indian Acad Neurol 2015;18:60-2.
3. Fajardo M, Kim SH, Szabo RM. Incidence of carpal tunnel release: Trends and implications within the United States ambulatory care setting. J Hand Surg Am 2012;37:1599-605.
4. Gelfman R, Melton LJ 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long-term trends in carpal tunnel syndrome. Neurology 2009;72:33-41.
5. Wainner RS, Fritz JM, Irrgang JJ, Delitto A, Allison S, Boninger ML. Development of a clinical prediction rule for the diagnosis of carpal tunnel syndrome. Arch Phys Med Rehabil 2005;86:609-18.
6. Huisstede BM, Randsdorp MS, van den Brink J, Franke TP, Koes BW, Hoogvliet P. Effectiveness of oral pain medication and corticosteroid injections for carpal tunnel syndrome: A systematic review. Arch Phys Med Rehabil 2018;99:1609-22.e10.
7. Huisstede BM, Hoogvliet P, Franke TP, Randsdorp MS, Koes BW. Carpal tunnel syndrome: Effectiveness of physical therapy and electrophysical modalities. An updated systematic review of randomized controlled trials. Arch Phys Med Rehabil 2018;99:1623-34.e23.
8. Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, van Middelkoop M, Koes BW. Carpal tunnel syndrome. Part I: Effectiveness of nonsurgical treatments-a systematic review. Arch Phys Med Rehabil 2010;91:981-1004.
9. Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. United States: Oxford University Press; 2001. p. 1024.
10. Preston DC, Shapiro B. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. United Kingdom: Butterworth-Heinemann; 2005. p. 704.
11. Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of ultrasound-guided carpal tunnel injection using in-plane ulnar approach: A prospective, randomized, single-blinded study. Medicine (Baltimore) 2014;93:e350.
12. Makhlouf T, Emil NS, Sibbitt WL Jr., Fields RA, Bankhurst AD. Outcomes and cost-effectiveness of carpal tunnel injections using sonographic needle guidance. Clin Rheumatol 2014;33:849-58.
13. Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically guided carpal tunnel injections: The ulnar approach. J Ultrasound Med 2008;27:1485-90.
14. Soyupek F, Yesildag A, Kutluhan S, Askin A, Ozden A, Uslusoy GA, et al. Determining the effectiveness of various treatment modalities in carpal tunnel syndrome by ultrasonography and comparing ultrasonographic findings with other outcomes. Rheumatol Int 2012;32:3229-34.
15. Klauser AS, Ellah MM, Halpern EJ, Siedentopf C, Auer T, Eberle G, et al. Sonographic cross-sectional area measurement in carpal tunnel syndrome patients: Can delta and ratio calculations predict severity compared to nerve conduction studies? Eur Radiol 2015;25:2419-27.
16. Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med 2018;12:e1480-8.
17. Malahias MA, Chytas D, Babis GC, Nikolaou VS. Platelet-rich plasma guided injections: Clinical application in peripheral neuropathies. Front Surg 2014;1:41.
18. Fnais N, Gomes T, Mahoney J, Alissa S, Mamdani M. Temporal trend of carpal tunnel release surgery: A population-based time series analysis. PLoS One 2014;9:e97499.
19. Graham B. Nonsurgical treatment of carpal tunnel syndrome. J Hand Surg Am 2009;34:531-4.
20. Ballestero-Perez R, Plaza-Manzano G, Urraca-Gesto A, Romo-Romo F, Atín-Arratibel ML, Pecos-Martín D, et al. Effectiveness of nerve gliding exercises on carpal tunnel syndrome: A systematic review. J Manipulative Physiol Ther 2017;40:50-9.
21. Roghani RS, Holisaz MT, Tarkashvand M, Delbari A, Gohari F, Boon AJ, et al. Different doses of steroid injection in elderly patients with carpal tunnel syndrome: A triple-blind, randomized, controlled trial. Clin Interv Aging 2018;13:117-24.
22. Sim SE, Gunasagaran J, Goh KJ, Ahmad TS. Short-term clinical outcome of orthosis alone vs combination of orthosis, nerve, and tendon gliding exercises and ultrasound therapy for treatment of carpal tunnel syndrome. J Hand Ther 2019;32:411-6.
23. Vahi PS, Kals M, Kõiv L, Braschinsky M. Preoperative corticosteroid injections are associated with worse long-term outcome of surgical carpal tunnel release. Acta Orthop 2014;85:102-6.
24. Kim SD. Efficacy of tendon and nerve gliding exercises for carpal tunnel syndrome: A systematic review of randomized controlled trials. J Phys Ther Sci 2015;27:2645-8.
25. Filius A, Scheltens M, Bosch HG, van Doorn PA, Stam HJ, Hovius SE, et al. Multidimensional ultrasound imaging of the wrist: Changes of shape and displacement of the median nerve and tendons in carpal tunnel syndrome. J Orthop Res 2015;33:1332-40.
26. Butler DS. The Sensitive Nervous System. Australia: Noigroup Publications; 2000. p. 431.
27. Lim YH, Chee DY, Girdler S, Lee HC. Median nerve mobilization techniques in the treatment of carpal tunnel syndrome: A systematic review. J Hand Ther 2017;30:397-406.
28. Bland JD, Ashworth NL. Does prior local corticosteroid injection prejudice the outcome of subsequent carpal tunnel decompression? J Hand Surg Eur Vol 2016;41:130-6.
29. Gupta S, Tewari AK, Nair V, Gupta A. Reliability of motor parameters for follow-up after local steroid injection in carpal tunnel syndrome. J Neurosci Rural Pract 2013;4:392-6.
30. Uzun H, Bitik O, Uzun O, Ersoy US, Aktaş E. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome. J Plast Surg Hand Surg 2017;51:301-5.
31. Malahias MA, Chytas D, Mavrogenis AF, Nikolaou VS, Johnson EO, Babis GC. Platelet-rich plasma injections for carpal tunnel syndrome: A systematic and comprehensive review. Eur J Orthop Surg Traumatol 2019;29:1-8.
32. Guven SC, Özçakar L, Kaymak B, Kara M, Akınci A. Short-term effectiveness of platelet-rich plasma in carpal tunnel syndrome: A controlled study. J Tissue Eng Regen Med 2019;13:709-14.
33. Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. Int J Dermatol 2014;53:109-13.
34. Harmon K, Hanson R, Bowen J. Section VIII: Platelet Rich Plasma (PRP) Guidelines; 2011. Available from: http://www.cellmedicinesociety.org.
35. Raimondo S, Fornaro M, Tos P, Battiston B, Giacobini-Robecchi MG, Geuna S. Perspectives in regeneration and tissue engineering of peripheral nerves. Ann Anat 2011;193:334-40.
36. Sanchez M, Anitua E, Delgado D, Sanchez P, Prado R, Orive G, et al. Platelet-rich plasma, a source of autologous growth factors and biomimetic scaffold for peripheral nerve regeneration. Expert Opin Biol Ther 2017;17:197-212.
37. Sanchez M, Yoshioka T, Ortega M, Delgado D, Anitua E. Ultrasound-guided platelet-rich plasma injections for the treatment of common peroneal nerve palsy associated with multiple ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc 2014;22:1084-9.
38. Yu W, Wang J, Yin J. Platelet-rich plasma: A promising product for treatment of peripheral nerve regeneration after nerve injury. Int J Neurosci 2011;121:176-80.
39. Kuo YC, Lee CC, Hsieh LF. Ultrasound-guided perineural injection with platelet-rich plasma improved the neurophysiological parameters of carpal tunnel syndrome: A case report. J Clin Neurosci 2017;44:234-6.
40. Malahias MA, Johnson EO, Babis GC, Nikolaou VS. Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen Res 2015;10:1856-9.
41. Raeissadat SA, Karimzadeh A, Hashemi M, Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Musculoskelet Disord 2018;19:49.
42. Wu YT, Ho TY, Chou YC, Ke MJ, Li TY, Huang GS, et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: A prospective randomized, single-blind controlled trial. Sci Rep 2017;7:94.
43. Bland JD. Hydrodissection for treatment of carpal tunnel syndrome. Muscle Nerve 2018;57:4-5.
44. Cass SP. Ultrasound-guided nerve hydrodissection: What is it? A review of the literature. Curr Sports Med Rep 2016;15:20-2.
45. Wu YT, Chen SR, Li TY, Ho TY, Shen YP, Tsai CK, et al. Nerve hydrodissection for carpal tunnel syndrome: A prospective, randomized, double-blind, controlled trial. Muscle Nerve 2019;59:174-80.
46. Evers S, Thoreson AR, Smith J, Zhao C, Geske JR, Amadio PC. Ultrasound-guided hydrodissection decreases gliding resistance of the median nerve within the carpal tunnel. Muscle Nerve 2018;57:25-32.
47. Tat J, Wilson KE, Keir PJ. Pathological changes in the subsynovial connective tissue increase with self-reported carpal tunnel syndrome symptoms. Clin Biomech (Bristol, Avon) 2015;30:360-5.
48. Wang Y, Filius A, Zhao C, Passe SM, Thoreson AR, An KN, et al. Altered median nerve deformation and transverse displacement during wrist movement in patients with carpal tunnel syndrome. Acad Radiol 2014;21:472-80.
49. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283-91.
50. Bakhsh H, Ibrahim I, Khan W, Smitham P, Goddard N. Assessment of validity, reliability, responsiveness and bias of three commonly used patient-reported outcome measures in carpal tunnel syndrome. Ortop Traumatol Rehabil 2012;14:335-40.
51. Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the boston carpal tunnel questionnaire. BMC Musculoskelet Disord 2006;7:78.
52. Sambandam SN, Priyanka P, Gul A, Ilango B. Critical analysis of outcome measures used in the assessment of carpal tunnel syndrome. Int Orthop 2008;32:497-504.
53. Ozyurekoglu T, McCabe SJ, Goldsmith LJ, LaJoie AS. The minimal clinically important difference of the carpal tunnel syndrome symptom severity scale. J Hand Surg Am 2006;31:733-8; discussion 739-40.
54. Ozer K, Malay S, Toker S, Chung KC. Minimal clinically important difference of carpal tunnel release in diabetic and nondiabetic patients. Plast Reconstr Surg 2013;131:1279-85.
55. Jacobson JA, Fundamentals of musculoskeletal ultrasound. 3rd ed. Fundamentals of Radiology Series. Philadelphia, PA: Elsevier; 2018. p. 459.
56. Shi Q, Bobos P, Lalone EA, Warren L, MacDermid JC. Comparison of the short-term and long-term effects of surgery and nonsurgical intervention in treating carpal tunnel syndrome: A systematic review and meta-analysis. Hand (N Y) 2020;15:13-22.
 |
 |
| Dr. Nathan J Savage | Dr. Joseph Albano |
| How to Cite This Article: Savage NJ, Albano J. Marrying Tendon and Nerve Gliding Exercises with Hydrodissection Following Injection for Carpal Tunnel Syndrome – A New Treatment Approach? Journal of Orthopaedic Case Reports 2020 December;10(9): 38-46. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




