[box type=”bio”] Learning Point of the Article: [/box]
Inion OTPS TM absorbable plates are not appropriate for the fixation of isolated ulnar diaphyseal fractures.
Case Report | Volume 10 | Issue 4 | JOCR July 2020 | Page 49-53 | Dimitrios Kapoutsis, Dimitrios Kitridis, Nikolaos Platon Sachinis, Avraam Ploumis, Panagiotis Givissis. DOI: 10.13107/jocr.2020.v10.i04.1798
Authors: Dimitrios Kapoutsis[1], Dimitrios Kitridis[1],[2], Nikolaos Platon Sachinis[2], Avraam Ploumis[2], Panagiotis Givissis[2]
[1]Department of Orthopaedics, 424 Army General Training Hospital, Thessaloniki, Greece,
[2]Department of Orthopaedics, George Papanikolaou Hospital, Aristotle University of Thessaloniki, Greece.
Address of Correspondence:
Dr. Dimitrios Kitridis,
424 Army General Training Hospital, Thessaloniki, Greece.
E-mail: dkitridis@gmail.com
Abstract
Introduction: Absorbable materials have been used as fixation devices in orthopaedic surgery. However, their use for treating isolated ulnar diaphyseal fractures in adults has not yet been studied. The aim of this study was to assess whether Inion OTPSTM absorbable implants consisting of L-lactide, D, L-lactide, and trimethylene carbonate provide adequate fixation for the healing of isolated ulnar diaphyseal fractures, their complication rate, and the patients’ clinical functional outcome.
Materials and Methods: Three consecutive patients (all women; mean age, 45 years, and range 38–55 years) with isolated, unstable ulnar fractures were enrolled and treated operatively using Inion OTPSTM absorbable plates and screws. Discontinuation of the study was decided because of the early failure of all implants. The patients were assessed clinically (DASH Score and grip strength) and radiographically at 6 weeks, 3 months, 6 months, and 9 months. The incidence of late foreign body reactions was evaluated for 10 years follow-up period.
Results: Implant failure was noticed radiographically in the early post-operative period in all three patients. Subsequently, one patient was treated operatively using metallic devices, and the other two with prolonged splinting. All fractures healed uneventfully in variable time frames. No foreign body reactions were noticed during and beyond the degradation period, other than a small painless mass in one case.
Conclusion: The results of the current study suggest that the Inion OTPSTM plating system is not appropriate for the fixation of isolated unstable ulnar diaphyseal fractures. It seems that these specific implants cannot withstand the internal mechanical forces of this anatomical area despite the protective splinting.
Keywords: Absorbable materials, absorbable plates, post-operative complications, ulnar fractures.
Introduction
Isolated ulnar diaphyseal fractures are relatively rare [1]; however, they have a high complication rate [1, 2]. When located in the distal two-thirds of the ulna, with <50% displacement and less than 10 degrees angulation, they may be treated non-operatively [3]. All other types are considered unstable and are treated operatively with internal fixation [1, 2, 3]. Although absorbable implants have been used as fixation devices, their use for treating isolated ulnar diaphyseal fractures in adults has not yet been studied [4, 5, 6, 7, 8]. The aim of this study was to assess the outcomes of fixation with third-generation Inion OTPSTM absorbable implants consisting of L-lactide, D, L-lactide, and trimethylene carbonate in a series of patients with isolated ulnar fractures. The mechanical stability of the implants, their complications, including late-presenting foreign body reactions, and the final clinical result were evaluated. This case series study was approved by the institution’s ethics committee (74/16-4-2005). From April 2005 to December 2006, three patients with displaced, isolated ulnar diaphyseal fractures were enrolled (mean age 45 years old) (Fig. 1). Patients with complex fractures of both radius and ulna, osteoporosis, a history of other pathological or hereditary bone disorders that affect bone metabolism, and smokers were excluded from the study. All patients provided written informed consent. In January 2007, both the orthopaedic team and the ethics committee decided discontinuation of the study, due to early implant failure and no added benefit to the patients.
Implants
Six-hole extended plates and 3.1-mm screws from the Inion OTPSTM Biodegradable Plating System (Inion, Tampere, Finland) were used. The implants are proprietary copolymers blends based on L-lactide, D, L-lactide, and trimethylene carbonate. According to the manufacturer, plates lose mechanical strength 18–36 weeks postoperatively; complete resorption is expected to take place in 2–4 years. These implants are licensed for the use for internal fixation and have been approved by several quality management system standards (ISO 9001, ISO 13485, EN 46001, FDA 510K).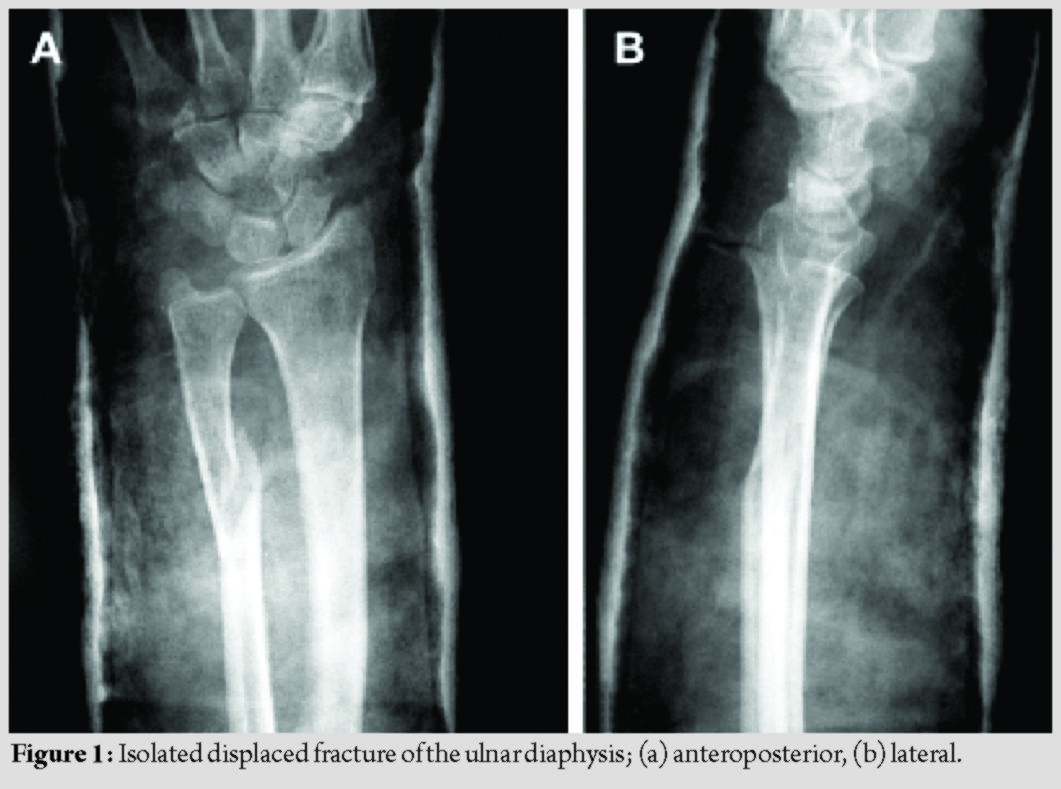
Operative procedure
All patients were operated by the senior author under general anesthesia and pneumatic tourniquet use. A longitudinal skin incision was done over the fracture site, with the patient supine, his arm on a hand table, and the elbow flexed at 90 degrees. After reduction, the fracture was stabilized with a lag screw when feasible, and the aforementioned plate was placed between the flexor carpi ulnaris and flexor carpi radialis, with three bicortical plate screws in each main fracture fragment (Fig. 2). No screw head breakage occurred during placement of the plate, for all patients.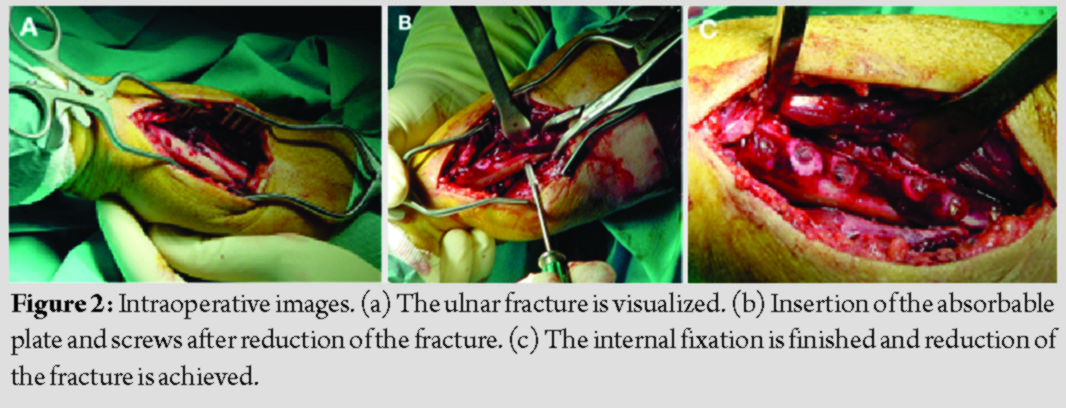
Post-operative protocol
Radiographs were obtained immediately postoperatively (Fig. 3). Sugar-tong splint immobilization was maintained for 6 weeks to avoid rotation of the forearm and the patients were advised to initiate hand rehabilitation exercises immediately postoperatively; elbow exercises were scheduled after removal of the aforementioned splint.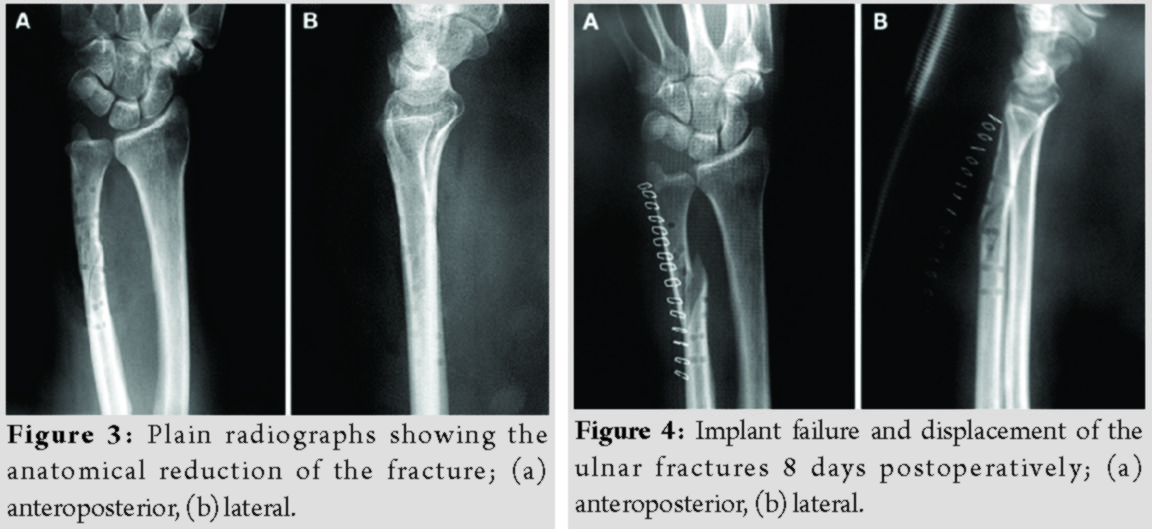
Outcomes
Follow-up assessments were scheduled at 6 weeks, 3 months, 6 months, and 9 months. The measurements were conducted by an independent investigator, who did not participate in any of the procedures. The active and passive range of motion, as well as grip strength of both arms using a hydraulic dynamometer was measured (Jamar, NexGen Ergonomics Inc, Quebec, Canada). The patients’ functional outcome was evaluated using the disabilities of the arm, Shoulder and Hand Score (DASH Score) [9]. To exclude the possibility of delayed foreign body reactions, the patients were reassessed for signs of inflammation in an annual base, for 10 consecutive years.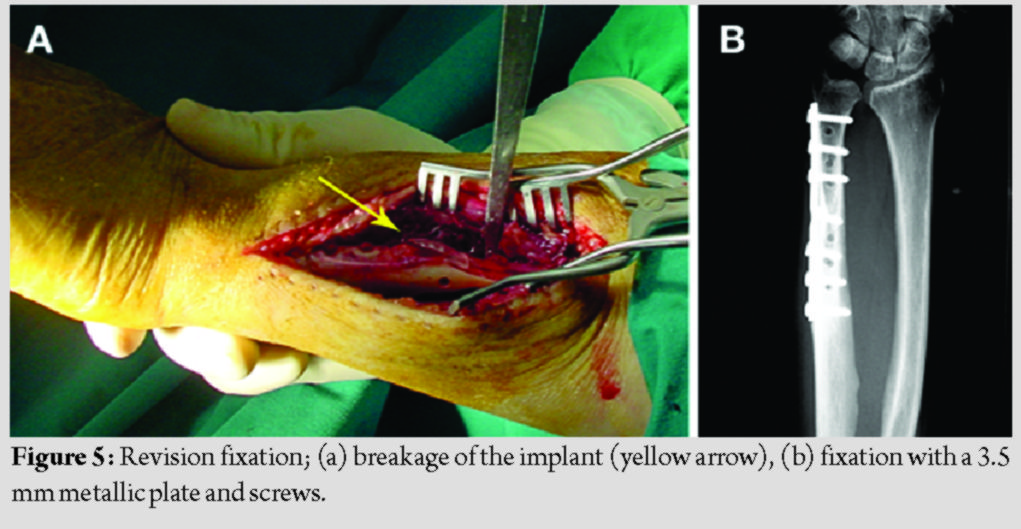
Results
The patients’ demographics and comorbidities are shown in Table 1. All three patients reported pain and unexpected swelling few days after the operation and the implants’ failure was confirmed radiologically (Fig. 4). 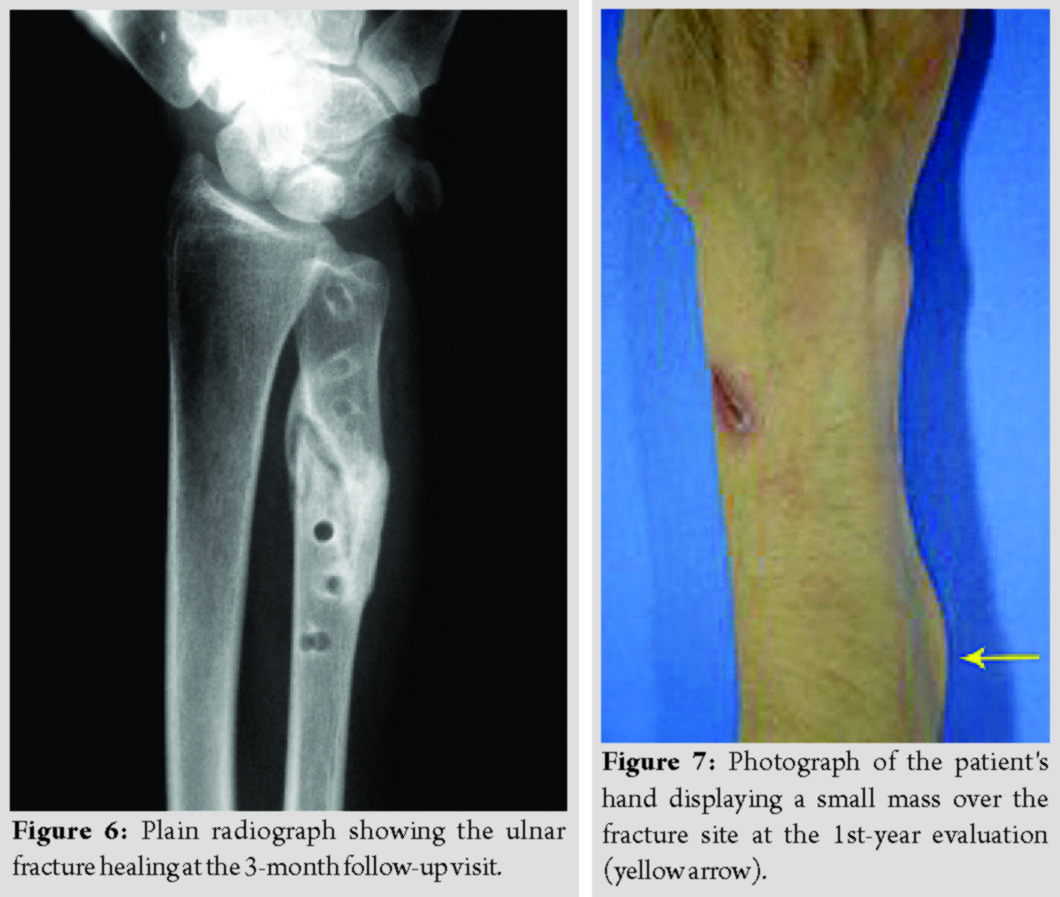 Reoperation using metallic implants was proposed as a definitive treatment for all patients. However, two of them denied it and were further treated with a plaster of Paris cast. The other patient, a 55 years old woman, underwent a revision fixation using a 3.5 metallic plate and screws.
Reoperation using metallic implants was proposed as a definitive treatment for all patients. However, two of them denied it and were further treated with a plaster of Paris cast. The other patient, a 55 years old woman, underwent a revision fixation using a 3.5 metallic plate and screws. 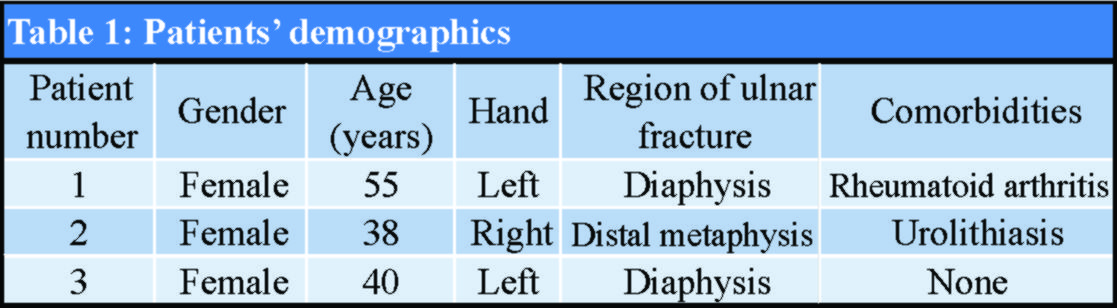 The fragmented absorbable plate was removed (Fig. 5), and a 3.5 mm metallic plate and screws were used for refixation. Subsequently, all fractures healed uneventfully (Fig. 6). The patients’ grip strength and DASH score at 9 months are presented in Table 2.
The fragmented absorbable plate was removed (Fig. 5), and a 3.5 mm metallic plate and screws were used for refixation. Subsequently, all fractures healed uneventfully (Fig. 6). The patients’ grip strength and DASH score at 9 months are presented in Table 2.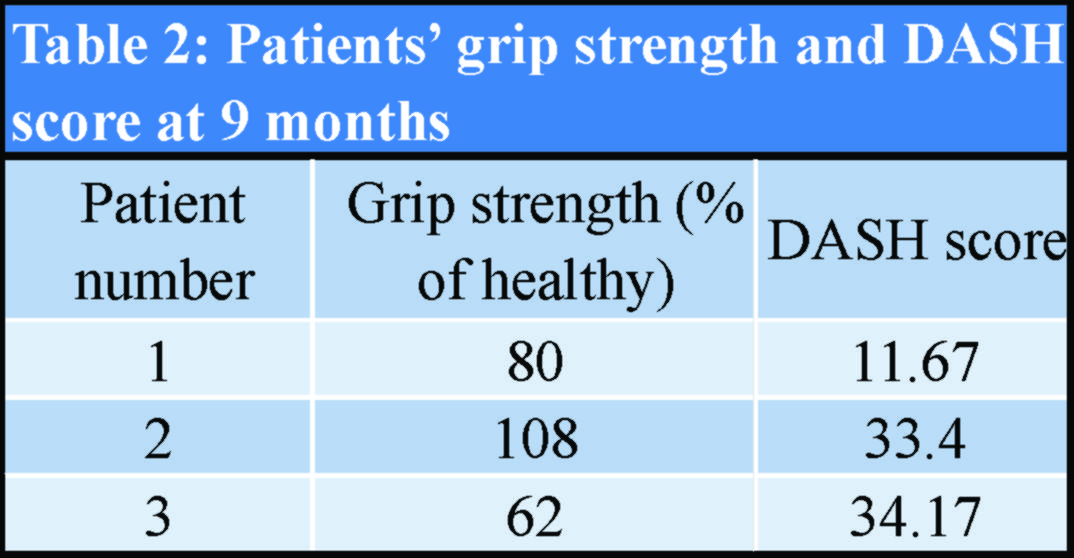
During the 10 years of clinical follow-up assessments, no severe soft-tissue reaction to the absorbable polymer materials was observed. In one patient, a small, palpable, painless mass over the fracture site was apparent at the 1st-year evaluation and remained at the same size since then (Fig. 7). Debridement was not decided because we did not notice any further signs of inflammation.
Discussion
The gold standard for treating displaced isolated ulnar diaphyseal fractures is open reduction and internal fixation with compression plates and screws [1, 2, 3]. Internal fixation using absorbable implants theoretically eliminates the need for implant removal operations and their potential complications [10, 11]. Although they have not gained widespread popularity among orthopedic surgeons, they still represent an area of continuing development [4, 5, 6, 7, 8, 12]. The outcomes of fixation with Inion OTPSTM absorbable plates and screws were assessed in a series of patients with isolated unstable ulnar diaphyseal fractures. The absorbable plates failed within the 1st month in all three cases, despite the protective splinting, showing that these plates cannot withstand the internal musculoligamentous mechanical forces of this particular area. According to the manufacturer, the elasticity modulus of self-reinforced absorbable polymers is close to that of cortical bone and higher than that of cancellous bone; the one-half of the initial bending and shear strength is lost after 18 weeks, which is an adequate time frame for bone healing per se [4, 13, 14]. The hypothesis that the sinusoidal movements of the elbow generate higher forces than the average may explain these problematic failures [15]. A potential solution to this problem would be to increase the thickness of the plates, but this could increase the foreign body reactions, which can be provoked with implant volumes that exceed the capacity of the surrounding soft tissues [16]. Recommendations regarding these products suggest using the minimum possible implant size to reduce the amount of breakdown products [17]. A major drawback of absorbable implants is that they cause foreign body reactions during and beyond the degradation process, which sometimes require surgical debridement [18, 19]. Studies have shown a lower rate of such complications with the use of modern third-generation L-lactide, D,L-lactide, and trimethylene carbonate implants [20, 21, 22, 23]. However, several case series still report foreign body reactions, using the same polylactic acid implants, even 2 years after the operation [24, 25]. In the current study, no significant reaction was observed during the 10 years follow-up, which exceeds the 4 years maximum degradation period. The only soft-tissue reaction was a small painless mass with no signs of inflammation in one of the patients. The strong point of the current study is that the constant failure of the implants implies that their use is inappropriate for the specific anatomical area. Another strong point is the long follow-up of the patients. On the other hand, the study has several limitations. The most important is the small number of patients, which does not allow proper analysis and statistically significant results. However, early discontinuation of the study was considered ethically correct, due to no added benefit to the patients. Another limitation is that a control group treated with metallic implants was not used. However, there is a sufficient body of published information proving that metallic plates currently constitute the gold standard [1, 2, 3]. The theoretical advantages offered by absorbable implants are the elimination of a removal operation and the reduction of the stress shielding effect, so the study outcomes focused on these issues [10, 11].
Conclusion
The results of the present study suggest that Inion OTPSTM implants are not appropriate for the fixation of isolated unstable ulnar diaphyseal fractures. Implant failure was noted in the early post-operative period in all our cases. It seems that these specific implants cannot withstand the internal musculoligamentous mechanical forces of this anatomical area despite the protective splinting. No significant soft-tissue reactions were observed during and beyond the degradation period, other than a small painless mass in one case.
Clinical Message
Inion OTPSTM absorbable plating system is not appropriate for the fixation of isolated unstable ulnar diaphyseal fractures. Early implant failure was noted in all three cases proving that the specific implants cannot withstand the internal musculoligamentous mechanical forces of this anatomical area despite the protective splinting. No severe soft-tissue reactions were observed during the follow-up period.
References
1. Handoll HH, Pearce P. Interventions for treating isolated diaphyseal fractures of the ulna in adults. Cochrane Database Syst Rev 2012;6:CD000523.
2. Mackay D, Wood L, Rangan A. The treatment of isolated ulnar fractures in adults: A systematic review. Injury 2000;31:565-70.
3. Sauder DJ, Athwal GS. Management of isolated ulnar shaft fractures. Hand Clin 2007;23:179-84.
4. Rokkanen PU, Böstman O, Hirvensalo E, Mäkelä EA, Partio EK, Pätiälä H, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials 2000;21:2607-13.
5. Van Manen CJ, Dekker ML, Van Eerten PV, Rhemrev SJ, Van Olden GD, Van Der Elst M. Bio-resorbable versus metal implants in wrist fractures: A randomised trial. Arch Orthop Trauma Surg 2008;128:1413-7.
6. Van Der Eng DM, Schep NW, Schepers T. Bioabsorbable versus metallic screw fixation for tibiofibular syndesmotic ruptures: A meta-analysis. J Foot Ankle Surg 2015;54:657-62.
7. Xie Y, Cai L, Deng Z, Ran B, Hu C. Absorbable screws versus metallic screws for distal tibiofibular syndesmosis injuries: A meta-analysis. J Foot Ankle Surg 2015;54:663-70.
8. Li ZH, Yu AX, Guo XP, Qi BW, Zhou M, Wang WY. Absorbable implants versus metal implants for the treatment of ankle fractures: A meta-analysis. Exp Ther Med 2013;5:1531-7.
9. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The upper extremity collaborative group (UECG). Am J Ind Med 1996;29:602-8.
10. Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000;21:2335-46.
11. Yao CK, Lin KC, Tarng YW, Chang WN, Renn JH. Removal of forearm plate leads to a high risk of refracture: Decision regarding implant removal after fixation of the forearm and analysis of risk factors of refracture. Arch Orthop Trauma Surg 2014;134:1691-7.
12. Kontakis GM, Pagkalos JE, Tosounidis TI, Melissas J, Katonis P. Bioabsorbable materials in orthopaedics. Acta Orthop Belg 2007;73:159-69.
13. Törmälä P. Biodegradable self-reinforced composite materials; manufacturing structure and mechanical properties. Clin Mater 1992;10:29-34.
14. Törmälä P, Pohjonen T, Rokkanen P. Bioabsorbable polymers: Materials technology and surgical applications. Proc Inst Mech Eng H 1998;212:101-11.
15. Joyce GC, Rack PM, Ross HF. The forces generated at the human elbow joint in response to imposed sinusoidal movements of the forearm. J Physiol 1974;240:351-74.
16. Weiler A, Helling HJ, Kirch U, Zirbes TK, Rehm KE. Foreign-body reaction and the course of osteolysis after polyglycolide implants for fracture fixation: Experimental study in sheep. J Bone Joint Surg Br 1996;78:369-76.
17. Wood GD. Inion biodegradable plates: The first century. Br J Oral Maxillofac Surg 2006;44:38-41.
18. Waris E, Ashammakhi N, Kaarela O, Raatikainen T, Vasenius J. Use of bioabsorbable osteofixation devices in the hand. J Hand Surg Br 2004;29:590-8.
19. Böstmann O. Intense granulomatous inflammatory lesions associated with absorbable internal fixation devices made of polyglycolide in ankle fractures. Clin Orthop Relat Res 1992;278:193-9.
20. Losken HW, Van Aalst JA, Mooney MP, Godfrey VL, Burt T, Teotia S, et al. Biodegradation of inion fast-absorbing biodegradable plates and screws. J Craniofac Surg 2008;19:748-56.
21. Nieminen T, Rantala I, Hiidenheimo I, Keränen J, Kainulainen H, Wuolijoki E, et al. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J Mater Sci Mater Med 2008;19:1155-63.
22. Mavrogenis AF, Kanellopoulos AD, Nomikos GN, Papagelopoulos PJ, Soucacos PN. Early experience with biodegradable implants in pediatric patients. Clin Orthop Relat Res 2009;467:1591-8.
23. Kukk A, Nurmi JT. A retrospective follow-up of ankle fracture patients treated with a biodegradable plate and screws. Foot Ankle Surg 2009;15:192-7.
24. Givissis PK, Stavridis SI, Papagelopoulos PJ, Antonarakos PD, Christodoulou AG. Delayed foreign-body reaction to absorbable implants in metacarpal fracture treatment. Clin Orthop Relat Res 2010;468:3377-83.
25. Rodrigo V, Maza A, Calatayud JB, Bances L, Diaz FJ, Gimeno MJ, et al. Long-term follow-up of anterior cervical discectomy and fusion with bioabsorbable plates and screws. Clin Neurol Neurosurg 2015;136:116-21.
 |
 |
 |
 |
 |
| Dr. Dimitrios Kapoutsis | Dr. Dimitrios Kitridis | Dr. Nikolaos Platon Sachinis | Dr. Avraam Ploumis | Dr. Panagiotis Givissis |
| How to Cite This Article: Kapoutsis D, Kitridis D, Sachinis NP, Ploumis A, Givissis P. Αbsorbable Plates for Isolated Ulnar Diaphyseal Fractures in Adults – A Case Series Study. Journal of Orthopaedic Case Reports 2020 July;10(4): 49-53. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com




